Ganciclovir
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ɡænˈsaɪkləvɪər/ |
| Trade names | Cytovene; Cymevene; Vitrasert; others |
| Other names | Ganciclovir sodium; gancyclovir; DHPG; 9-(1,3-dihydroxy-2-propoxymethyl)guanine |
IUPAC name
| |
| Clinical data | |
| Main uses | Treat and prevent cytomegalovirus (CMV), herpetic keratitis[1][2] |
| Side effects | Low white blood cells, low platelets, low red blood cells, fever, diarrhea, increased sweating, headache, kidney problems[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous, eye drop, by mouth, intravitreal |
| External links | |
| AHFS/Drugs.com | Systemic: Monograph Eye drop: Monograph |
| MedlinePlus | a605011 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 5% (by mouth) |
| Metabolism | guanylate kinase (CMV UL97 gene product) |
| Elimination half-life | 2.5–5 hours |
| Excretion | Kidney |
| Chemical and physical data | |
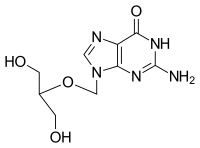
| Formula | C9H13N5O4 |
| Molar mass | 255.234 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 250 °C (482 °F) (dec.) |
SMILES
| |
InChI
| |
Ganciclovir, sold under the brand name Cytovene among others, is an antiviral medication used to treat and prevent cytomegalovirus disease (CMV).[1] This includes CMV retinitis, pneumonitis, encephalitis, and congenital CMV disease.[3] It may also be used for certain VZV infections of the eye.[3] It is given by injection into a vein or the eye.[1] It may also be used as eye drops.[2]
Common side effects when given by injection include low white blood cells, low platelets, low red blood cells, fever, diarrhea, increased sweating, headache, and kidney problems.[3] Other side effects may include infertility and cancer.[3] Use in pregnancy may harm the baby.[3] It is a nucleoside analog of guanine.[3]
Ganciclovir was patented in 1980 and approved for medical use in 1988.[4] In the United Kingdom a 500 mg vial costs the NHS about £30 as of 2021.[1] This amount in the United States is about 70 USD.[5]
Medical use

Ganciclovir is indicated for:[6]
- Sight-threatening CMV retinitis in severely immunocompromised people
- CMV pneumonitis in bone marrow transplant recipients
- Prevention of CMV disease in bone marrow and solid organ transplant recipients
- Confirmed CMV retinitis in people with AIDS (intravitreal implant)
It is also used for acute CMV colitis in HIV/AIDS and CMV pneumonitis in immunosuppressed patients.
Ganciclovir has also been used with some success in treating Human herpesvirus 6 infections.[7]
Ganciclovir has also been found to be an effective treatment for herpes simplex virus epithelial keratitis.[8]
Dosage
By injection it is given at a dose of 5 mg/kg once to twice per day.[1]
Acute infections are treated in two phases:
- induction phase, 5 mg per kilogram intravenously every 12 hours for 14–21 days, the intravenous dose given as a 1-hour infusion
- maintenance phase, 5 mg per kg intravenously every day
Stable disease is treated with 1000 mg orally three times daily. Similar dosing is used to prevent disease in high-risk patients, such as those infected with human immunodeficiency virus (HIV) or those with organ transplants.
Ganciclovir is also available in slow-release formulations for insertion into the vitreous humour of the eye, as treatment for CMV retinitis (associated with HIV infection).
A topical eye gel is approved for the treatment of acute herpes simplex keratitis.[2]
Side effects
Ganciclovir is associated with a range of serious haematological side effects. Common side effects (≥1% of people) include: granulocytopenia, neutropenia, anaemia, thrombocytopenia, fever, nausea, vomiting, dyspepsia, diarrhea, abdominal pain, flatulence, anorexia, raised liver enzymes, headache, confusion, hallucination, seizures, pain and phlebitis at injection site (due to high pH), sweating, rash, itch, increased serum creatinine and blood urea concentrations.[6]
Toxicity
Ganciclovir is considered a potential human carcinogen, teratogen, and mutagen. It is also considered likely to cause inhibition of spermatogenesis. Thus, it is used judiciously and handled as a cytotoxic drug in the clinical setting.[6][9]
Mechanism of action
Ganciclovir is a synthetic analogue of 2′-deoxy-guanosine. It is first phosphorylated to ganciclovir monophosphate by a viral kinase encoded by the cytomegalovirus (CMV) gene UL97 during infection. Subsequently, cellular kinases catalyze the formation of ganciclovir diphosphate and ganciclovir triphosphate, which is present in 10-fold greater concentrations in CMV or herpes simplex virus (HSV)-infected cells than uninfected cells.
Ganciclovir triphosphate is a competitive inhibitor of deoxyguanosine triphosphate (dGTP) incorporation into DNA and preferentially inhibits viral DNA polymerases more than cellular DNA polymerases. In addition, ganciclovir triphosphate serves as a poor substrate for chain elongation, thereby disrupting viral DNA synthesis by a second route.
Pharmacokinetics
Absorption of the oral form is very limited—about 5% fasting, about 8% with food. It achieves a concentration in the central nervous system of about 50% of the plasma level. About 90% of plasma ganciclovir is eliminated unchanged in the urine, with a half-life of 2–6 hours, depending on renal function (elimination takes over 24 hours in end-stage renal disease).
See also
Valganciclovir, the prodrug of ganciclovir
References
- 1 2 3 4 5 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 676. ISBN 978-0857114105.
- 1 2 3 "Ganciclovir Monograph for Professionals". Drugs.com. Archived from the original on 5 March 2021. Retrieved 2 December 2021.
- 1 2 3 4 5 6 7 "Ganciclovir Sodium Monograph for Professionals". Drugs.com. Archived from the original on 4 May 2017. Retrieved 2 December 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 504. ISBN 9783527607495. Archived from the original on 2016-12-20. Retrieved 2021-06-28.
- ↑ "Ganciclovir Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 2 December 2021.
- 1 2 3 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ Nakano (2009). "Detection and identification of U69 gene mutations encoded by ganciclovir-resistant human herpesvirus 6 using denaturing high-performance liquid chromatography".
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Wilhelmus KR (January 2015). "Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis". The Cochrane Database of Systematic Reviews. 1: CD002898. doi:10.1002/14651858.CD002898.pub5. PMC 4443501. PMID 25879115.
- ↑ Roche Products Pty Ltd. Cymevene (Australian Approved Product Information). Dee Why (NSW): Roche; 2005.
External links
- "Ganciclovir". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-05-04. Retrieved 2021-06-28.
- "Ganciclovir sodium". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-09-26. Retrieved 2021-06-28.
- Noble S, Faulds D (July 1998). "Ganciclovir. An update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients". Drugs. 56 (1): 115–46. doi:10.2165/00003495-199856010-00012. PMID 9664203.
- Spector SA (1999). "Oral ganciclovir". Advances in Experimental Medicine and Biology. 458: 121–7. doi:10.1007/978-1-4615-4743-3_11. ISBN 978-1-4613-7150-2. PMID 10549384.
{{cite journal}}: Cite journal requires|journal=(help) - Couchoud-Heyer C (July 2007). "WITHDRAWN: Cytomegalovirus prophylaxis with antiviral agents for solid organ transplantation". The Cochrane Database of Systematic Reviews (4): CD001320. doi:10.1002/14651858.cd001320.pub2. PMID 17636667.
| Identifiers: |
|---|