Tecovirimat
 | |
| Names | |
|---|---|
| Trade names | Tpoxx |
| Other names | ST-246 |
IUPAC name
| |
| Clinical data | |
| Main uses | Smallpox, monkeypox, cowpox, complications of smallpox vaccination[1] |
| Side effects | Nausea, headache, abdominal pain[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 600 mg BID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Chemical and physical data | |
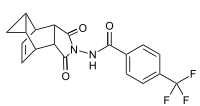
| Formula | C19H15F3N2O3 |
| Molar mass | 376.335 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Tecovirimat, sold under the brand name Tpoxx, is an antiviral medication with activity against smallpox.[2] Other uses may include monkeypox, cowpox, and complications of smallpox vaccination.[1] It is taken by mouth.[2]
Common side effects include nausea, headache, and abdominal pain.[2] No serious side effects have been seen.[4] It works by blocking orthopoxvirus VP37 protein, which prevents the virus from leave a cell.[2]
Tecovirimat became the first drug approved for use in smallpox in the United States in 2018.[5] Two million doses are in the US Strategic National Stockpile in the event that smallpox reoccurs.[6] As of 2021 it is not approved in Europe.[1]
Medical uses
Tecovirimat was first used for treatment in December 2018 after a laboratory-acquired vaccinia virus infection.[7]
Evidence
The results of trials support its use against smallpox and other related orthopoxviruses. It has shown potential for a variety of uses including preventive healthcare, as a post-exposure therapeutic, as a therapeutic and an adjunct to vaccination.[8]
Tecovirimat can be taken orally and has recently been permitted for Phase II trials by the U.S. Food and Drug Administration (FDA). In Phase I trials, tecovirimat was generally well tolerated with no serious adverse events.[9]
Dosage
In those over 40 kg it is taken at a dose of 600 mg twice per day for two weeks.[2]
Mechanism of action
Tecovirimat inhibits the function of a major envelope protein required for the production of extracellular virus. The drug prevents the virus from leaving an infected cell, hindering the spread of the virus within the body.[10]
History
Based on research on monkeypox-challenged prairie dogs and safety studies in humans, Tecovirimat became the first drug approved for use in smallpox in the United States in 2018.[5] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[11]
Due to its importance for biodefense, the FDA has designated tecovirimat for 'fast-track' status, creating a path for expedited FDA review and eventual regulatory approval. On 13 July 2018, the FDA announced approval of tecovirimat.[12]
Society and culture
Originally researched by the National Institute of Allergy and Infectious Diseases, the drug was previously owned by Viropharma and discovered in collaboration with scientists at USAMRIID. It is owned and manufactured by Siga Technologies, a pharmaceutical company in the biodefense arena that won a government contract to develop the drug.
References
- 1 2 3 "Tecovirimat". SPS - Specialist Pharmacy Service. 17 July 2018. Archived from the original on 31 July 2020. Retrieved 24 September 2021.
- 1 2 3 4 5 6 7 "Tecovirimat Monograph for Professionals". Drugs.com. Archived from the original on 28 November 2021. Retrieved 24 September 2021.
- ↑ "U.S. Food and Drug Administration Approves SIGA Technologies' TPOXX (tecovirimat) for the Treatment of Smallpox - SIGA". SIGA. Archived from the original on 21 September 2018. Retrieved 1 July 2021.
- ↑ McNeil Jr DG. "Drug to Treat Smallpox Approved by F.D.A., a Move Against Bioterrorism". The New York Times. Archived from the original on 28 March 2019. Retrieved 16 July 2018.
- 1 2 Bonville, Cynthia; Domachowske, Joseph (2021). "28. Smallpox". In Domachowske, Joseph; Suryadevara, Manika (eds.). Vaccines: A Clinical Overview and Practical Guide. Switzerland: Springer. p. 337. ISBN 978-3-030-58416-0. Archived from the original on 3 June 2022. Retrieved 1 June 2022.
- ↑ Cunningham A (13 July 2018). "FDA approves the first smallpox treatment". Archived from the original on 12 July 2018. Retrieved 1 July 2021.
- ↑ Whitehouse ER, Rao AK, Yu YC, et al. Novel Treatment of a Vaccinia Virus Infection from an Occupational Needlestick — San Diego, California, 2019. MMWR Morb Mortal Wkly Rep 2019;68:943–946. DOI:10.15585/mmwr.mm6842a2
- ↑ "Siga Technologies". Archived from the original on 20 February 2012. Retrieved 1 July 2021.
- ↑ Jordan R, Tien D, Bolken TC, Jones KF, Tyavanagimatt SR, Strasser J, et al. (May 2008). "Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor". Antimicrobial Agents and Chemotherapy. 52 (5): 1721–7. doi:10.1128/AAC.01303-07. PMC 2346641. PMID 18316519.
- ↑ Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, et al. (October 2005). "An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge". Journal of Virology. 79 (20): 13139–49. doi:10.1128/JVI.79.20.13139-13149.2005. PMC 1235851. PMID 16189015.
- ↑ New Drug Therapy Approvals 2018 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2019. Archived from the original on 17 September 2020. Retrieved 16 September 2020.
- ↑ Commissioner, Office of the. "Press Announcements - FDA approves the first drug with an indication for treatment of smallpox". U.S. Food and Drug Administration (FDA). Archived from the original on 23 April 2019. Retrieved 1 July 2021.
External links
- "Tecovirimat". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 1 May 2021. Retrieved 1 July 2021.
- "Tecovirimat monohydrate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 29 August 2021. Retrieved 1 July 2021.
| Identifiers: |
|---|