Smallpox vaccine
 Smallpox vaccine diluent in a syringe alongside a vial of dried Dryvax smallpox vaccine and bifurcated needle. | |
| Vaccine description | |
|---|---|
| Type | Live virus |
| Names | |
| Trade names | ACAM2000, Imvanex, Jynneos, others |
| Other names | Monkeypox vaccine |
| Clinical data | |
| Main uses | Smallpox, monkeypox[1][2] |
| Side effects | Pain at injection site, tiredness, muscle pain, headache[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | Subcutaneous |
| External links | |
| AHFS/Drugs.com | ACAM2000 Monograph MVA-BN Monograph |
| Legal | |
| License data | |
| Legal status | |
Smallpox vaccines are vaccines that prevents smallpox and Mpox.[1][2] For smallpox it was about 95% effective for 3 to 5 years.[1] For monkeypox it has been at least 85% effective.[4] While it is not routinely given to prevent smallpox, it is available for potential exposures.[5] In 2022, it was recommended for people at high risk of these diseases.[4] Older vaccines are given by scarification while newer ones are given by injection under the skin.[3][2]
Current vaccines include ACAM2000, which was approved for medical use in the United States in 2007 and MVA-BN which was approved in 2019.[3][2] ACAM2000 is a live vaccine that can duplicate itself, while MVA-BN is a live vaccine that cannot duplicate itself.[4] Other types are under investigation.[5]
Common side effects of ACAM2000 include swollen lymph nodes, tiredness, fever, and rash at the site of inoculation.[3] Severe side effects may include myocarditis, eczema vaccinatum, and encephalitis.[3] Use in pregnancy may harm the baby.[3] Common side effects of MVA-BN include pain at the site of injection, tiredness, muscle pain, and headache.[2] Severe side effects may include anaphylaxis.[2] There is no evidence of increased harm in pregnancy or eczema.[2]
Smallpox vaccine was first applied in a scientific manner in 1796, when Edward Jenner demonstrated that infection by the relatively mild cowpox virus conferred protection from smallpox.[6] Cowpox; however, had been used previously in 1774 by Benjamin Jesty and other physicians in the 1700s but had not been promoted to the same degree.[6] The practice of vaccination become common across Europe by 1800.[6] From 1966 to 1977, the World Health Organization conducted a vaccination campaign that eradicated smallpox, making it the only human disease to be eradicated.[6][7] In the 2000s the cost of a replicating vaccine was about 5 USD per dose while that of a non-replicating vaccine was about 29 USD.[8]
Types
ACAM2000
ACAM2000 is a smallpox vaccine which was approved for use in the United States in 2007.[10] It contains live vaccinia virus, cloned from the same strain used in the earlier vaccine, Dryvax. While the Dryvax virus was cultured in the skin of calves and freeze-dried, ACAM2000s virus is cultured in kidney epithelial cells (Vero cells) from an African green monkey. Efficacy and rates of side effects are similar to Dryvax.[11] The vaccine is not routinely available to the US public; however, it is used in the military and maintained in the Strategic National Stockpile.[12][13]
A droplet of ACAM2000 is administered via the skin (scarification) using 15 jabs of a bifurcated needle. ACAM2000 should not be injected by the intradermal, subcutaneous, intramuscular, or intravenous routes.[14]
MVA-BN
MVA-BN (sold under the brand names Imvanex, Imvamune, and Jynneos)[12][15] is a live, non-replicating vaccine for smallpox and monkeypox derived from Modified Vaccinia Ankara, an attenuated strain of the vaccinia virus. It was approved in the European Union in adults in 2013,[16] and was approved in Canada.[17][18][19] MVA-BN was approved in the United States in 2019.[20][21]
MVA-BN contains Modified vaccinia Ankara, a attenuated form of the vaccinia virus that does not replicate in human cells and hence does not cause the sometimes serious side effects that are seen with replicating smallpox vaccines (i.e. preparations of unattenuated vaccinia virus). These replicating vaccines use different strains of the vaccinia virus, which all replicate in humans, and are not recommended for people with immune deficiencies and exfoliative skin disorders, such as eczema or atopic dermatitis. Vaccines containing vaccinia viruses were used effectively in the campaign to eradicate smallpox. Because of similarities between vaccinia and the smallpox virus, the antibodies produced against vaccinia have been shown to protect against smallpox. In contrast to replicating smallpox vaccines, which are applied by scarification using a bifurcated needle, MVA-BN is administered by injection via the subcutaneous route.[22]
As of August 2014, 24 million doses of MVA-BN were delivered to the U.S. Strategic National Stockpile (SNS) for use by people with weakened immune systems or atopic dermatitis.[12]
Prior vaccines
Dryvax was a freeze-dried live-virus smallpox vaccine prepared from calf lymph and used for smallpox eradication efforts.[12] The vaccine was effective, providing successful immunogenicity in about 95% of vaccinated persons. Dryvax has seriousside-effects in about 1% to 2% of cases.[11] Before its discontinuation, it was the world's oldest smallpox vaccine, created in the late 1800s by American Home Products, a predecessor of Wyeth. By the 1940s, Wyeth was the leading U.S. manufacturer of the vaccine and the only manufacturer by the 1960s. After world health authorities declared smallpox had been eradicated from nature in 1980, Wyeth stopped making the vaccine.[23] The U.S. Centers for Disease Control and Prevention (CDC) kept a stockpile for use in case of emergency. In 2003 this supply helped contain an outbreak of monkeypox in the United States.[24] In February 2008, the CDC disposed of the last of its 12 million doses of Dryvax. Its supply was replaced by ACAM2000,[10] a more modern product manufactured in laboratories by Acambis, a division of Sanofi Pasteur.[23][25] Trace amounts of the antibiotics (added during processing) may have been present in Dryvax: neomycin sulfate, chlortetracycline hydrochloride, polymyxin B sulfate, and dihydrostreptomycin sulfate.[26]
Calf lymph was the name given to a type of smallpox vaccine used in the 19th century, and which was manufactured up to the 1970s.[27] Calf lymph was known as early as 1805 in Italy,[28] but it was the Lyon Medical Conference of 1864 which made the technique known to the wider world.[29] In 1898 calf lymph became the standard method of vaccination for smallpox in the United Kingdom, when arm-to-arm vaccination was eventually banned[30][31] (due to complications such as the simultaneous transmission of syphilis, erysipelas or smallpox itself).[32]
Safety
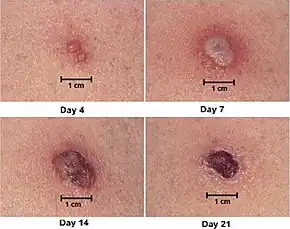
Some types of vaccine are infectious, which improves its effectiveness, but can cause serious complications in people with impaired immune systems (for example those on chemotherapy or with AIDS or eczema), and is not yet considered safe for pregnant women. Vaccines that only contain attenuated vaccinia viruses (an attenuated virus is one in which the pathogenicity has been decreased through serial passage) have been proposed, but some researchers have questioned the possible effectiveness of such a vaccine. According to the Centers for Disease Control and Prevention (CDC), "vaccination within 3 days of exposure will prevent or significantly lessen the severity of smallpox symptoms in the vast majority of people. Vaccination 4 to 7 days after exposure likely offers some protection from disease or may modify the severity of disease." This, along with vaccinations of so-called first responders, is the current plan of action being devised by the United States Department of Homeland Security (including Federal Emergency Management Agency) in the United States.
Among US military service members vaccinated between December 2002 and March 2003 18 cases of probable myopericarditis were reported, a rate of 7.8 per 100,000 over 30 days.[33] Starting in early 2003, the United States government started a program to vaccinate 500,000 volunteer health care professionals throughout the country. Recipients were healthcare workers who would be first-line responders in the event of a bioterrorist attack. Some healthcare workers refused, worried about side effects, and healthcare systems refused to participate. Fewer than 40,000 actually received the vaccine.[34]
In 2007, the Vaccines and Related Biological Products Advisory Committee of the U.S. Food and Drug Administration (FDA) voted unanimously that a new live virus vaccine, ACAM2000, is both safe and effective for use in persons at high risk of exposure to smallpox virus. However, due to the high rate of serious side effects, the vaccine will only be made available to the CDC (a part of the United States Department of Health and Human Services) for the Strategic National Stockpile.[35]
History
Variolation
The mortality of the severe form of smallpox – variola major – was very high without vaccination, up to 35% in some outbreaks.[36] A method of inducing immunity known as inoculation, insufflation or "variolation" was practiced before the development of a modern vaccine and likely occurred in Africa and China well before the practice arrived in Europe.[37] It may also have occurred in India, but this is disputed; other investigators contend the ancient Sanskrit medical texts of India do not describe these techniques.[37][38] The first clear reference to smallpox inoculation was made by the Chinese author Wan Quan (1499–1582) in his Douzhen xinfa (痘疹心法) published in 1549.[39] Inoculation for smallpox does not appear to have been widespread in China until the reign era of the Longqing Emperor (r. 1567–1572) during the Ming Dynasty.[40] In China, powdered smallpox scabs were blown up the noses of the healthy. The patients would then develop a mild case of the disease and from then on were immune to it. The technique did have a 0.5–2.0% mortality rate, but that was considerably less than the 20–30% mortality rate of the disease itself. Two reports on the Chinese practice of inoculation were received by the Royal Society in London in 1700; one by Dr. Martin Lister who received a report by an employee of the East India Company stationed in China and another by Clopton Havers.[41] According to Voltaire (1742), the Turks derived their use of inoculation from neighbouring Circassia. Voltaire does not speculate on where the Circassians derived their technique from, though he reports that the Chinese have practiced it "these hundred years".[42]
Variolation was also practiced throughout the latter half of the 17th century by physicians in Turkey, Persia, and Africa. In 1714 and 1716, two reports of the Ottoman Empire Turkish method of inoculation were made to the Royal Society in England, by Emmanuel Timoni, a doctor affiliated with the British Embassy in Constantinople,[43] and Giacomo Pylarini. Source material tells us on Lady Mary Wortley Montagu; "When Lady Mary was in the Ottoman Empire, she discovered the local practice of inoculation against smallpox called variolation."[44] In 1718 she had her son, aged five variolated. He recovered quickly. She returned to London and had her daughter variolated in 1721 by Charles Maitland, during an epidemic of smallpox. This encouraged the British Royal Family to take an interest and a trial of variolation was carried out on prisoners in Newgate Prison. This was successful and in 1722 Caroline of Ansbach, the Princess of Wales, allowed Maitland to vaccinate her children.[45] The success of these variolations assured the British people that the procedure was safe.[43]
—Dr. Peter Kennedy
Stimulated by a severe epidemic, variolation was first employed in North America in 1721. The procedure had been known in Boston since 1706, when preacher Cotton Mather learned it from Onesimus, a man he held as a slave, who – like many of his peers – had been inoculated in Africa before they were kidnapped.[47] This practice was widely criticized at first.[48] However, a limited trial showed six deaths occurred out of 244 who were variolated (2.5%), while 844 out of 5980 died of natural disease (14%), and the process was widely adopted throughout the colonies.[49]
The inoculation technique was documented as having a mortality rate of only one in a thousand. Two years after Kennedy's description appeared, March 1718, Dr. Charles Maitland successfully inoculated the five-year-old son of the British ambassador to the Turkish court under orders from the ambassador's wife Lady Mary Wortley Montagu, who four years later introduced the practice to England.[50]
An account from letter by Lady Mary Wortley Montagu to Sarah Chiswell, dated 1 April 1717, from the Turkish Embassy describes this treatment:
The small-pox so fatal and so general amongst us is here entirely harmless by the invention of ingrafting (which is the term they give it). There is a set of old women who make it their business to perform the operation. Every autumn in the month of September, when the great heat is abated, people send to one another to know if any of their family has a mind to have the small-pox. They make parties for this purpose, and when they are met (commonly fifteen or sixteen together) the old woman comes with a nutshell full of the matter of the best sort of small-pox and asks what veins you please to have opened. She immediately rips open that you offer to her with a large needle (which gives you no more pain than a common scratch) and puts into the vein as much venom as can lye upon the head of her needle, and after binds up the little wound with a hollow bit of shell, and in this manner opens four or five veins. … The children or young patients play together all the rest of the day and are in perfect health till the eighth. Then the fever begins to seize them and they keep their beds two days, very seldom three. They have very rarely above twenty or thirty in their faces, which never mark, and in eight days time they are as well as before the illness. … There is no example of any one that has died in it, and you may believe I am very well satisfied of the safety of the experiment since I intend to try it on my dear little son. I am patriot enough to take pains to bring this useful invention into fashion in England, and I should not fail to write to some of our doctors very particularly about it if I knew any one of them that I thought had virtue enough to destroy such a considerable branch of their revenue for the good of mankind, but that distemper is too beneficial to them not to expose to all their resentment the hardy wight that should undertake to put an end to it. Perhaps if I live to return I may, however, have courage to war with them.[51]
Early vaccination

In the early empirical days of vaccination, before Louis Pasteur's work on establishing the germ theory and Joseph Lister's on antisepsis and asepsis, there was considerable cross-infection. William Woodville, one of the early vaccinators and director of the London Smallpox Hospital is thought to have contaminated the cowpox matter – the vaccine – with smallpox matter and this essentially produced variolation. Other vaccine material was not reliably derived from cowpox, but from other skin eruptions of cattle.[52]
During the earlier days of empirical experimentation in 1758, American Calvinist Jonathan Edwards died from a smallpox inoculation. Some of the earliest statistical and epidemiological studies were performed by James Jurin in 1727 and Daniel Bernoulli in 1766.[53] In 1768 Dr John Fewster reported that variolation induced no reaction in persons who had had cowpox.[54][55]

Edward Jenner was born in Berkeley, England as an orphan. As a young child, Jenner was variolated with the other schoolboys through parish funds, but nearly died due to the seriousness of his infection. Fed purgative medicine and going through the bloodletting process, Jenner was put in one of the variolation stables until he recovered.[56] At the age of 13, he was apprenticed to apothecary Daniel Ludlow and later surgeon George Hardwick in nearby Sodbury. He observed that people who caught cowpox while working with cattle were known not to catch smallpox. Jenner assumed a causal connection but the idea was not taken up at that time. From 1770 to 1772 Jenner received advanced training in London at St Georges Hospital and as the private pupil of John Hunter, then returned to set up practice in Berkeley.[57]
Perhaps there was already an informal public understanding of some connection between disease resistance and working with cattle. The "beautiful milkmaid" seems to have been a frequent image in the art and literature of this period. But it is known for certain that in the years following 1770, at least six people in England and Germany (Sevel, Jensen, Jesty 1774, Rendall, Plett 1791) tested successfully the possibility of using the cowpox vaccine as an immunization for smallpox in humans.[58]
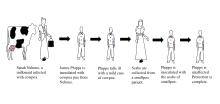
Jenner sent a paper reporting his observations to the Royal Society in April 1797. It was not submitted formally and there is no mention of it in the Society's records. Jenner had sent the paper informally to Sir Joseph Banks, the Society's president, who asked Everard Home for his views. Reviews of his rejected report, published for the first time in 1999, were skeptical and called for further vaccinations.[59] Additional vaccinations were performed and in 1798 Jenner published his work entitled An Inquiry into the Causes and Effects of the Variolae Vaccinae, a disease discovered in some of the western counties of England, particularly Gloucestershire and Known by the Name of Cow Pox.[37][60][61] It was an analysis of 23 cases including several individuals who had resisted natural exposure after previous cowpox. It is not known how many Jenner vaccinated or challenged by inoculation with smallpox virus; e.g. Case 21 included 'several children and adults'. Crucially all of at least four whom Jenner deliberately inoculated with smallpox virus resisted it. These included the first and last patients in a series of arm-to-arm transfers. He concluded that cowpox inoculation was a safe alternative to smallpox inoculation, but rashly claimed that the protective effect was lifelong. This last proved to be incorrect.[62] Jenner also tried to distinguish between 'True' cowpox which produced the desired result and 'Spurious' cowpox which was ineffective and/or produced severe reaction. Modern research suggests Jenner was trying to distinguish between effects caused by what would now be recognised as non-infectious vaccine, a different virus (e.g. paravaccinia/milker's nodes), or contaminating bacterial pathogens. This caused confusion at the time, but would become important criteria in vaccine development.[63] A further source of confusion was Jenner's belief that fully effective vaccine obtained from cows originated in an equine disease, which he mistakenly referred to as grease. This was criticised at the time but vaccines derived from horsepox were soon introduced and later contributed to the complicated problem of the origin of vaccinia virus, the virus in present-day vaccine.[64]: 165–78
The introduction of the vaccine to the New World took place in Trinity, Newfoundland, in 1798 by Dr. John Clinch, boyhood friend and medical colleague of Jenner.[65][66] The first smallpox vaccine in the United States was administered in 1799. The physician Valentine Seaman gave his children a smallpox vaccination using a serum acquired from Jenner.[67][68] By 1800, Jenner's work had been published in all the major European languages and had reached Benjamin Waterhouse in the United States – an indication of rapid spread and deep interest.[69]: 262–67 Despite some concern about the safety of vaccination the mortality using carefully selected vaccine was close to zero, and it was soon in use all over Europe and the United States.[70][71]
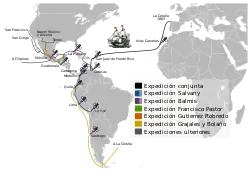
In 1804 the Balmis Expedition, an official Spanish mission commanded by Francisco Javier de Balmis, sailed to spread the vaccine throughout the Spanish Empire, first to the Canary Islands and on to Spanish Central America. While his deputy, José Salvany, took vaccine to the west and east coasts of Spanish South America, Balmis sailed to Manila in the Philippines and on to Canton and Macao on the Chinese coast. He returned to Spain in 1806.[72]
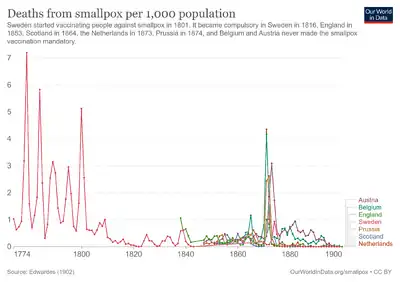
Contrary to popular belief, the first state to introduce compulsory vaccinations was the Principality of Lucca and Piombino on September 25, 1806.[73] On August 26, 1807, Bavaria introduced a similar measure. Baden followed in 1809, Prussia in 1815, Württemberg in 1818, Sweden in 1816, England in 1867 and the German Empire in 1874 through the Reichs Vaccination Act.[74][75] In Lutheran Sweden, the Protestant clergy played a pioneering role in voluntary smallpox vaccination as early as 1800.[76] The first vaccination was carried out in Liechtenstein in 1801, and from 1812 it was mandatory to vaccinate.[77]
The question of who first tried cowpox inoculation/vaccination cannot be answered with certainty. Most, but still limited, information is available for Benjamin Jesty, Peter Plett and John Fewster. In 1774 Jesty, a farmer of Yetminster in Dorset, observing that the two milkmaids living with his family were immune to smallpox, inoculated his family with cowpox to protect them from smallpox. He attracted a certain amount of local criticism and ridicule at the time then interest waned. Attention was later drawn to Jesty, and he was brought to London in 1802 by critics jealous of Jenner's prominence at a time when he was applying to Parliament for financial reward.[78] During 1790–92 Peter Plett, a teacher from Holstein, reported limited results of cowpox inoculation to the Medical Faculty of the University of Kiel. However, the Faculty favoured variolation and took no action.[79] John Fewster, a surgeon friend of Jenner's from nearby Thornbury, discussed the possibility of cowpox inoculation at meetings as early as 1765. He may have done some cowpox inoculations in 1796 at about the same time that Jenner vaccinated Phipps. However, Fewster, who had a flourishing variolation practice, may have considered this option but used smallpox instead. He thought vaccination offered no advantage over variolation, but maintained friendly contact with Jenner and certainly made no claim of priority for vaccination when critics attacked Jenner's reputation.[80] It seems clear that the idea of using cowpox instead of smallpox for inoculation was considered, and actually tried in the late 18th century, and not just by the medical profession. Therefore, Jenner was not the first to try cowpox inoculation. However, he was the first to publish his evidence and distribute vaccine freely, provide information on selection of suitable material, and maintain it by arm-to-arm transfer. The authors of the official World Health Organization (WHO) account Smallpox and its Eradication assessing Jenner's role wrote:[49]: 264
Publication of the Inquiry and the subsequent energetic promulgation by Jenner of the idea of vaccination with a virus other than variola virus constituted a watershed in the control of smallpox for which he, more than anyone else deserves the credit.
As vaccination spread, some European countries made it compulsory. Concern about its safety led to opposition and then repeal of legislation in some instances.[80]: 236–40 [81] Compulsory infant vaccination was introduced in England by the 1853 Vaccination Act. By 1871, parents could be fined for non-compliance, and then imprisoned for non-payment.[81]: 202–13 This intensified opposition, and the 1898 Vaccination Act introduced a conscience clause. This allowed exemption on production of a certificate of conscientious objection signed by two magistrates. Such certificates were not always easily obtained and a further Act in 1907 allowed exemption by a statutory declaration which could not be refused. Although theoretically still compulsory, the 1907 Act effectively marked the end of compulsory infant vaccination in England.[81]: 233–38

In the United States vaccination was regulated by individual states, the first to impose compulsory vaccination being Massachusetts in 1809. There then followed sequences of compulsion, opposition and repeal in various states. By 1930 Arizona, Utah, North Dakota and Minnesota prohibited compulsory vaccination, 35 states allowed regulation by local authorities, or had no legislation affecting vaccination, whilst in ten states, including Washington, D.C. and Massachusetts, infant vaccination was compulsory.[69]: 292–93 Compulsory infant vaccination was regulated by only allowing access to school for those who had been vaccinated.[82] Those seeking to enforce compulsory vaccination argued that the public good overrode personal freedom, a view supported by the U.S. Supreme Court in Jacobson v. Massachusetts in 1905, a landmark ruling which set a precedent for cases dealing with personal freedom and the public good.[83]
Louis T. Wright,[84] an African-American Harvard Medical School graduate (1915), introduced, while serving in the Army during World War I, intradermal, smallpox vaccination for the soldiers.[85]
Manufacturing
Until the end of the 19th century, vaccination was performed either directly with vaccine produced on the skin of calves or, particularly in England, with vaccine obtained from the calf but then maintained by arm-to-arm transfer;[86] initially in both cases vaccine could be dried on ivory points for short-term storage or transport but increasing use was made of glass capillary tubes for this purpose towards the end of the century.[87] During this period there were no adequate methods for assessing the safety of the vaccine and there were instances of contaminated vaccine transmitting infections such as erysipelas, tetanus, septicaemia and tuberculosis.[63] In the case of arm-to-arm transfer there was also the risk of transmitting syphilis. Although this did occur occasionally, estimated as 750 cases in 100 million vaccinations,[88] some critics of vaccination e.g. Charles Creighton believed that uncontaminated vaccine itself was a cause of syphilis.[89] Smallpox vaccine was the only vaccine available during this period, and so the determined opposition to it initiated a number of vaccine controversies that spread to other vaccines and into the 21st century.
Sydney Arthur Monckton Copeman, an English Government bacteriologist interested in smallpox vaccine investigated the effects on the bacteria in it of various treatments, including glycerine. Glycerine was sometimes used simply as a diluent by some continental vaccine producers. However, Copeman found that vaccine suspended in 50% chemically-pure glycerine and stored under controlled conditions contained very few "extraneous" bacteria and produced satisfactory vaccinations.[90] He later reported that glycerine killed the causative organisms of erysipelas and tuberculosis when they were added to the vaccine in "considerable quantity", and that his method was widely used on the continent.[86] In 1896, Copeman was asked to supply "extra good calf vaccine" to vaccinate the future Edward VIII.[91]
Vaccine produced by Copeman's method was the only type issued free to public vaccinators by the English Government Vaccine Establishment from 1899. At the same time the 1898 Vaccination Act banned arm-to-arm vaccination, thus preventing transmission of syphilis by this vaccine. However, private practitioners had to purchase vaccine from commercial producers.[92] Although proper use of glycerine reduced bacterial contamination considerably the crude starting material, scraped from the skin of infected calves, was always heavily contaminated and no vaccine was totally free from bacteria. A survey of vaccines in 1900 found wide variations in bacterial contamination. Vaccine issued by the Government Vaccine Establishment contained 5,000 bacteria per gram, while commercial vaccines contained up to 100,000 per gram.[93] The level of bacterial contamination remained unregulated until the Therapeutic Substances Act, 1925 set an upper limit of 5,000 per gram, and rejected any batch of vaccine found to contain the causative organisms of erysipelas or wound infections.[63] Unfortunately glycerolated vaccine soon lost its potency at ambient temperatures which restricted its use in tropical climates. However, it remained in use into the 1970s where a satisfactory cold chain was available. Animals continued to be widely used by vaccine producers during the smallpox eradication campaign. A WHO survey of 59 producers, some of whom used more than one source of vaccine, found that 39 used calves, 12 used sheep and 6 used water buffalo, whilst only 3 made vaccine in cell culture and 3 in embryonated hens' eggs.[49]: 543–45 English vaccine was occasionally made in sheep during World War I but from 1946 only sheep were used.[87]
In the late 1940s and early 1950s, Leslie Collier, an English microbiologist working at the Lister Institute of Preventive Medicine, developed a method for producing a heat-stable freeze-dried vaccine in powdered form.[94][95] Collier added 0.5% phenol to the vaccine to reduce the number of bacterial contaminants but the key stage was to add 5% peptone to the liquid vaccine before it was dispensed into ampoules. This protected the virus during the freeze drying process. After drying the ampoules were sealed under nitrogen. Like other vaccines, once reconstituted it became ineffective after 1–2 days at ambient temperatures. However, the dried vaccine was 100% effective when reconstituted after 6 months storage at 37 °C (99 °F) allowing it to be transported to, and stored in, remote tropical areas. Collier's method was increasingly used and, with minor modifications, became the standard for vaccine production adopted by the WHO Smallpox Eradication Unit when it initiated its global smallpox eradication campaign in 1967, at which time 23 of 59 manufacturers were using the Lister strain.[49]: 545, 550
In a letter about landmarks in the history of smallpox vaccine, written to and quoted from by Derrick Baxby, Donald Henderson, chief of the Smallpox Eradication Unit from 1967–77 wrote; "Copeman and Collier made an enormous contribution for which neither, in my opinion ever received due credit".[96]
Smallpox vaccine was inoculated by scratches into the superficial layers of the skin, with a wide variety of instruments used to achieve this. They ranged from simple needles to multi-pointed and multi-bladed spring-operated instruments specifically designed for the purpose.[97]
A major contribution to smallpox vaccination was made in the 1960s by Benjamin Rubin, an American microbiologist working for Wyeth Laboratories. Based on initial tests with textile needles with the eyes cut off transversely half-way he developed the bifurcated needle. This was a sharpened two-prong fork designed to hold one dose of reconstituted freeze-dried vaccine by capillarity.[9] Easy to use with minimum training, cheap to produce ($5 per 1000), using one quarter as much vaccine as other methods, and repeatedly re-usable after flame sterilization, it was used globally in the WHO Smallpox Eradication Campaign from 1968.[49]: 472–73, 568–72 Rubin estimated that it was used to do 200 million vaccinations per year during the last years of the campaign.[9] Those closely involved in the campaign were awarded the "Order of the Bifurcated Needle". This, a personal initiative by Donald Henderson, was a lapel badge, designed and made by his daughter, formed from the needle shaped to form an "O". This represented "Target Zero", the objective of the campaign.[98]
Eradication of smallpox

Smallpox was eradicated by a massive international search for outbreaks, backed up with a vaccination program, starting in 1967. It was organised and co-ordinated by a World Health Organization (WHO) unit, set up and headed by Donald Henderson. The last case in the Americas occurred in 1971 (Brazil), south-east Asia (Indonesia) in 1972, and on the Indian subcontinent in 1975 (Bangladesh). After two years of intensive searches, what proved to be the last endemic case anywhere in the world occurred in Somalia, in October 1977.[49]: 526–37 A Global Commission for the Certification of Smallpox Eradication chaired by Frank Fenner examined the evidence from, and visited where necessary, all countries where smallpox had been endemic. In December 1979 they concluded that smallpox had been eradicated; a conclusion endorsed by the WHO General Assembly in May 1980.[49]: 1261–62 However, even as the disease was being eradicated there still remained stocks of smallpox virus in many laboratories. Accelerated by two cases of smallpox in 1978, one fatal (Janet Parker), caused by an accidental and unexplained containment breach at a laboratory at the University of Birmingham Medical School, the WHO ensured that known stocks of smallpox virus were either destroyed or moved to safer laboratories. By 1979, only four laboratories were known to have smallpox virus. All English stocks held at St Mary's Hospital, London were transferred to more secure facilities at Porton Down and then to the U.S. at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia in 1982, and all South African stocks were destroyed in 1983. By 1984, the only known stocks were kept at the CDC in the U.S. and the State Research Center of Virology and Biotechnology (VECTOR) in Koltsovo, Russia.[49]: 1273–76 These states report that their repositories are for possible anti-bioweaponry research and insurance if some obscure reservoir of natural smallpox is discovered in the future.
Origin
The exact origin of the modern smallpox vaccine is unclear.[99] Edward Jenner had obtained his vaccine from the cow, so he named the virus vaccinia, after the Latin word for cow. Jenner believed that both cowpox and smallpox were viruses that originated in the horse and passed to the cow.[100]: 52–53 Some doctors followed up on this speculation by inoculating humans with horsepox.[101] The situation was further muddied when Louis Pasteur developed techniques for creating vaccines in the laboratory in the late 19th century. As medical researchers subjected viruses to serial passage, inadequate recordkeeping resulted in the creation of laboratory strains with unclear origins.[64]: 4
By the early 20th century, the origins of the smallpox vaccine were muddled.[64] It was unknown whether the vaccine originated in smallpox, horsepox, or cowpox.[102] A number of competing hypotheses existed within the medical and scientific community. Some believed that Edward Jenner's cow had been accidentally inoculated with smallpox.[103] Others believed that smallpox and vaccinia shared a common ancestor.[104]: 64 In 1939, Allan Watt Downie showed that the vaccinia virus was serologically distinct from the "spontaneous" cowpox virus.[105] This work established vaccinia and cowpox as two separate viral species. The term vaccinia now refers only to the smallpox vaccine,[106] while cowpox no longer has a Latin name.[107]
The development of whole genome sequencing in the 1990s made it possible to build a phylogenetic tree of the orthopoxviruses. The vaccinia strains are most similar to each other, followed by horsepox and rabbitpox. Vaccinia's nearest cowpox relatives are the strains found in Russia, Finland, and Austria. Out of 20 cowpox strains that have been sequenced, the cowpox strains found in Great Britain are the least related to vaccinia.[108] However, the exact origin of vaccinia remains unclear. While rabbitpox is known to be a laboratory strain of vaccinia, the connection between vaccinia and horsepox is still debated. Some scientists think that horsepox is the ancestral strain that evolved into vaccinia.[109][101] Horsepox was believed to have become extinct in the wild by 2013,[110] but scientists controversially synthesized horsepox in 2017.[111][112]
Society and culture
Terminology
The term "vaccine" is from the Latin word for cow, reflecting the believed origin of smallpox vaccination. Edward Jenner referred to cowpox as variolae vaccinae (smallpox of the cow). The origins of the smallpox vaccine became murky over time,[113] especially after Louis Pasteur developed laboratory techniques for creating vaccines in the 19th century. Allan Watt Downie demonstrated in 1939 that the modern smallpox vaccine was serologically distinct from cowpox,[105] and vaccinia was subsequently recognized as a separate viral species. Whole-genome sequencing has revealed that vaccinia is most closely related to horsepox, and the cowpox strains found in Great Britain are the least closely related to vaccinia.[108]
The word "vaccine" is derived from Variolae vaccinae (i.e. smallpox of the cow), the term devised by Jenner to denote cowpox and used in the long title of his An enquiry into the causes and effects of Variolae vaccinae, known by the name of cow pox.[62] Vaccination, the term which soon replaced cowpox inoculation and vaccine inoculation, was first used in print by Jenner's friend, Richard Dunning in 1800.[57] Initially, the terms vaccine/vaccination referred only to smallpox, but in 1881 Louis Pasteur proposed that to honour Jenner the terms be widened to cover the new protective inoculations being introduced.
Stockpiles
Since smallpox has been eradicated, the public is not routinely vaccinated against the disease. The World Health Organization maintained a stockpile of 200 million doses in 1980 to guard against reemergence of the disease, but 99% of the stockpile was destroyed in the late 1980s when smallpox failed to return.[114] After the September 11 attacks in 2001, many governments began building up vaccine stockpiles again for fear of bioterrorism. Several companies sold off their stockpiles of vaccines manufactured in the 1970s, and production of smallpox vaccines resumed.[115] Aventis Pasteur discovered a stockpile from the 1950s and donated it to the U.S. government.[116]
Stockpiles of newer vaccines must be repurchased periodically since they carry expiration dates. The United States had received 269 million doses of ACAM2000 and 28 million doses of MVA-BN by 2019,[117][118] but only 100 million doses of ACAM2000 and 65,000 doses of MVA-BN were still available from the stockpile at the start of the 2022 monkeypox outbreak.[119] First-generation vaccines have no specified expiration date and remain viable indefinitely in deep freeze. The U.S. stockpile of WetVax was manufactured in 1956–1957 and maintained since then at -4 °F (-20 °C),[120] and it was still effective when tested in 2004.[121] Replicating vaccines also remain effective even at 1:10 dilution, so a limited number of doses can be stretched to cover a much larger population.[121]
| Country, region, or organization | Year | Doses (millions) | Composition |
|---|---|---|---|
| 2013 | 2.7 |
| |
(pledged) | 2018 | 27 | Various (1st, 2nd, 3rd)[122][123] |
| 2006 | 55 | 55 million Pourquier (1st)[124] | |
| 2022 | 100 | ||
| 2022 | 5[125] | ||
| 2006 | 56 | LC16m8 (3rd)[126] | |
| 2017 | ? | Lister/Elstree-RVIM (2nd)[49]: 588–589 | |
| 2022 | 35 | Lancy-Vaxina (1st)[127][128] | |
| 2022 | 185 |
Canada
In 2015 Canada was to have more than 100,000 doses of MVA-BN in stockpiles.[129] They were to receive 31 million USD more in 2022 and 2023.[130] At the prior reported cost of 29 USD per dose this could be about a million doses.[8] In 2022 the federal government delivered 70,000 doses to the provinces to use to prevent monkeypox.[131] In 2022 56 million USD more has been ordered, with 250,000 doses to arrived in 2023 and 250,000 doses to arrive in 2024.[132][133]
In 2020 Canada purchased 580,000 doses of ACAM2000 to arrive between March 2021 and March 2023, with the option to purchase 460,000 more doses by March 2024.[134]
References
- 1 2 3 "Vaccine Basics | Smallpox | CDC". www.cdc.gov. 15 February 2019. Archived from the original on 20 May 2022. Retrieved 26 July 2022.
- 1 2 3 4 5 6 7 8 9 "DailyMed - JYNNEOS- vaccinia virus modified strain ankara-bavarian nordic non-replicating antigen injection, suspension". dailymed.nlm.nih.gov. Archived from the original on 27 May 2022. Retrieved 26 July 2022.
- 1 2 3 4 5 6 "DailyMed - ACAM2000 (smallpox- vaccinia vaccine, live injection, powder, lyophilized, for solution". dailymed.nlm.nih.gov. Archived from the original on 24 May 2022. Retrieved 26 July 2022.
- 1 2 3 "Monkeypox and Smallpox Vaccine Guidance | Monkeypox | Poxvirus | CDC". www.cdc.gov. 18 July 2022. Archived from the original on 19 May 2022. Retrieved 26 July 2022.
- 1 2 Bonville, Cynthia; Domachowske, Joseph (2021). "28. Smallpox". In Domachowske, Joseph; Suryadevara, Manika (eds.). Vaccines: A Clinical Overview and Practical Guide. Switzerland: Springer. pp. 337–342. ISBN 978-3-030-58416-0. Archived from the original on 3 June 2022. Retrieved 11 June 2022.
- 1 2 3 4 Riedel, S (January 2005). "Edward Jenner and the history of smallpox and vaccination". Proceedings (Baylor University. Medical Center). 18 (1): 21–5. doi:10.1080/08998280.2005.11928028. PMID 16200144.
- ↑ "Smallpox vaccines". www.who.int. Archived from the original on 13 June 2020. Retrieved 26 July 2022.
- 1 2 Lambert de Rouvroit, Axel; Heegaard, Erik D. (January 2016). "Total costs associated with replicating and non-replicating smallpox vaccines". Global Security: Health, Science and Policy. 1 (1): 3–9. doi:10.1080/23793406.2016.1171944.
- 1 2 3 Rubin BA (May 1980). "A note on the development of the bifurcated needle for smallpox vaccination". WHO Chronicle. 34 (5): 180–81. PMID 7376638.
- 1 2 "ACAM2000". U.S. Food and Drug Administration (FDA). 20 September 2018. STN BL 125158. Archived from the original on 17 October 2019. Retrieved 16 October 2019.
- 1 2 Metzger W, Mordmueller BG (July 2007). "Vaccines for preventing smallpox". The Cochrane Database of Systematic Reviews (3): CD004913. doi:10.1002/14651858.CD004913.pub2. PMC 6532594. PMID 17636779.
- 1 2 3 4 "Smallpox Vaccine Supply & Strength". National Institute of Allergy and Infectious Diseases (NIAID). 26 September 2019. Archived from the original on 17 October 2019. Retrieved 16 October 2019.
- ↑ FDA/CBER Questions and Answers; Medication Guide; accessed 1 March 2008.
- ↑ Emergent Product Development Gaithersburg Inc. "Highlights of Prescribing Information: ACAM2000, (Smallpox (Vaccinia) Vaccine, Live)" (PDF). U.S. Food and Drug Administration. Archived (PDF) from the original on 25 April 2019. Retrieved 9 April 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Greenberg RN, Hay CM, Stapleton JT, Marbury TC, Wagner E, Kreitmeir E, et al. (2016). "A Randomized, Double-Blind, Placebo-Controlled Phase II Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN®) in 56–80-Year-Old Subjects". PLOS ONE. 11 (6): e0157335. Bibcode:2016PLoSO..1157335G. doi:10.1371/journal.pone.0157335. PMC 4915701. PMID 27327616.
- ↑ "Imvanex EPAR". European Medicines Agency (EMA). Archived from the original on 27 April 2022. Retrieved 2 October 2014.
- ↑ "Smallpox vaccine: Canadian Immunization Guide". Public Health Agency of Canada. January 2014. Archived from the original on 20 July 2020. Retrieved 24 June 2020.
- ↑ "Register of Innovative Drugs" (PDF). Health Canada. June 2020. Archived (PDF) from the original on 26 June 2020. Retrieved 24 June 2020. Lay summary.
{{cite web}}: Cite uses deprecated parameter|lay-url=(help) - ↑ "Products for Human Use. Submission #144762". Register of Innovative Drugs. Health Canada. Archived from the original on 17 June 2014. Retrieved 30 October 2014.
- ↑ "Jynneos". U.S. Food and Drug Administration (FDA). 24 September 2019. STN 125678. Archived from the original on 17 October 2019. Retrieved 16 October 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "FDA approves first live, non-replicating vaccine to prevent smallpox and monkeypox". U.S. Food and Drug Administration (FDA). 24 September 2019. Archived from the original on 17 October 2019. Retrieved 17 October 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Summary Basis for Regulatory Action Template". FDA. U.S. Food & Drug Administration. Archived from the original on 8 October 2021. Retrieved 8 October 2021.
- 1 2 "CDC to Destroy Oldest Smallpox Vaccine". Associated Press. 3 March 2008. Archived from the original on 16 November 2021. Retrieved 26 August 2019.
- ↑ Anderson MG, Frenkel LD, Homann S, and Guffey J. (2003), "A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values"; Pediatr Infect Dis J;22(12): 1093–96; discussion 1096–98.
- ↑ "Safety Surveillance Study of ACAM2000 Vaccinia Vaccine". clinicaltrials.gov. 29 January 2019. Archived from the original on 20 May 2022. Retrieved 16 October 2019.
- ↑ Wyeth package insert / U.S. Food and Drug Administration
- ↑ BMA (1905). Facts about Smallpox and Vaccination. British Medical Association. p. 21. Archived from the original on 16 November 2021. Retrieved 7 January 2021.
- ↑ Galbiati G (1810). Memoria Sulla Inoculazione Vaccina coll'Umore Ricavato Immediatement dalla Vacca Precedentemente Inoculata. Napoli.
- ↑ Congrès Medical de Lyon (1864). "Compterendu des travaux et des discussions". Gazette Med Lyon. 19: 449–71.
- ↑ Brown E (1902). The Case for vaccination. Baillière, Tindall & Cox. pp. 8, 21.
- ↑ Ballard E (1868). On Vaccination: Its Value and Alleged Dangers. London.
- ↑
Didgeon JA (May 1963). "Development of Smallpox Vaccine in England in the Eighteenth and Nineteenth Centuries". British Medical Journal. 1 (5342): 1367–72. doi:10.1136/bmj.1.5342.1367. PMC 2124036. PMID 20789814. - ↑ Halsell, Jeffrey S. (25 June 2003). "Myopericarditis Following Smallpox Vaccination Among Vaccinia-Naive US Military Personnel". JAMA. 289 (24): 3283–3289. doi:10.1001/jama.289.24.3283. ISSN 0098-7484. PMID 12824210. Archived from the original on 20 May 2022. Retrieved 20 May 2022.
- ↑ Mackenzie D (22 August 2003). "US smallpox vaccination plan grinds to a halt". New Scientist. Archived from the original on 7 February 2022. Retrieved 9 April 2022.
- ↑ "Vaccines and Related Biological Products Advisory Committee Meeting". U.S. Food and Drug Administration (FDA). 17 May 2007. Archived from the original on 20 October 2017. Retrieved 2 May 2013.
- ↑ Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 525–28. ISBN 978-0-8385-8529-0.
- 1 2 3 Riedel S (January 2005). "Edward Jenner and the history of smallpox and vaccination". Proceedings. 18 (1): 21–25. doi:10.1080/08998280.2005.11928028. PMC 1200696. PMID 16200144.
- ↑ Van Alphen J, Aris A (1995). "Medicine in India". Oriental Medicine: An Illustrated Guide to the Asian Arts of Healing. London: Serindia Publications. pp. 19–38. ISBN 978-0-906026-36-6.
- ↑ Needham J (1999). "Part 6, Medicine". Science and Civilization in China: Volume 6, Biology and Biological Technology. Cambridge: Cambridge University Press. p. 134.
- ↑ Temple R (1986). The Genius of China: 3,000 Years of Science, Discovery, and Invention. New York: Simon and Schuster. p. 137. ISBN 978-0-671-62028-8.
- ↑ Silverstein AM (2009). A History of Immunology (2nd ed.). Academic Press. p. 293. ISBN 9780080919461. Archived from the original on 3 August 2020. Retrieved 9 April 2022..
- ↑ Voltaire (1742). "Letter XI". Letters on the English. Archived from the original on 16 October 2018. Retrieved 9 April 2022.
- 1 2 Behbehani AM (December 1983). "The smallpox story: life and death of an old disease". Microbiological Reviews. 47 (4): 455–509. doi:10.1128/MMBR.47.4.455-509.1983. PMC 281588. PMID 6319980.
- ↑ Aboul-Enein BH, Ross MW, Aboul-Enein FH (2012). "Smallpox inoculation and the Ottoman contribution: A Brief Historiography" (PDF). Texas Public Health Journal. 64 (1): 12. Archived (PDF) from the original on 11 October 2021. Retrieved 9 April 2022.
- ↑ Livingstone, N. 2015. The Mistresses of Cliveden. Three centuries of scandal, power and intrigue (p. 229)
- ↑ Kennedy P (1715). An Essay on External Remedies Wherein it is Considered, Whether all the curable Distempers incident to Human Bodies, may not be cured by Outward Means. London: A. Bell.
- ↑ Willoughby B (12 February 2004). "Black History Month II: Why Wasn't I Taught That?". Tolerance in the News. Archived from the original on 14 January 2009. Retrieved 4 December 2008.
- ↑ "Open Collections Program: Contagion, The Boston Smallpox Epidemic, 1721". Archived from the original on 26 July 2018. Retrieved 27 August 2008.
- 1 2 3 4 5 6 7 8 9 Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID (1988). Smallpox and Its Eradication (PDF). History of International Public Health. Geneva: World Health Organization. ISBN 978-92-4-156110-5. Archived (PDF) from the original on 19 February 2015. Retrieved 5 November 2013.
- ↑ Robertson P (1974). The book of firsts. New York: C. N. Potter : distributed by Crown Publishers. ISBN 978-0-517-51577-8.
- ↑ "Montagu, Turkish Embassy Letters". Archived from the original on 15 April 2013. Retrieved 4 December 2008.
- ↑ "Statue of Dr Edward Jenner near the Italian Fountains, Kensington Gardens". lachlan.bluehaze.com.au. Archived from the original on 28 March 2006. Retrieved 16 October 2019.
- ↑ Blower S, Bernoulli D (2004). "An attempt at a new analysis of the mortality caused by smallpox and of the advantages of inoculation to prevent it. 1766" (PDF). Reviews in Medical Virology. 14 (5): 275–88. doi:10.1002/rmv.443. PMID 15334536. S2CID 8169180. Archived from the original (PDF) on 27 September 2007.
- ↑ Pearson G, ed. (1798). An inquiry concerning the history of the cowpox, principally with a view to supersede and extinguish the smallpox. London, England: J. Johnson. pp. 102–104. Archived from the original on 25 January 2022. Retrieved 9 April 2022.
- ↑ Thurston L, Williams G (2015). "An examination of John Fewster's role in the discovery of smallpox vaccination" (PDF). The Journal of the Royal College of Physicians of Edinburgh. 45 (2): 173–79. doi:10.4997/JRCPE.2015.217. PMID 26181536. Archived from the original on 16 July 2017. Retrieved 9 April 2022.
- ↑ Michael J. Bennett, War against Smallpox: Edward Jenner and the Global Spread of Vaccination (Cambridge, United Kingdom: Cambridge University Press, 2020), 32.
- 1 2 Bailey I (May 1996). "Edward Jenner (1749–1823): naturalist, scientist, country doctor, benefactor to mankind". Journal of Medical Biography. 4 (2): 63–70. doi:10.1177/096777209600400201. PMID 11616266. S2CID 30318738.
- ↑ Hammarsten JF, Tattersall W, Hammarsten JE (1979). "Who discovered smallpox vaccination? Edward Jenner or Benjamin Jesty?". Transactions of the American Clinical and Climatological Association. 90: 44–55. PMC 2279376. PMID 390826.
- ↑ Baxby D (January 1999). "Edward Jenner's unpublished cowpox inquiry and the Royal Society: Everard Home's report to Sir Joseph Banks". Medical History. 43 (1): 108–10. doi:10.1017/S0025727300064747. PMC 1044113. PMID 10885136.
- ↑ Winkelstein W (1992). "Not just a country doctor: Edward Jenner, scientist". Epidemiologic Reviews. 14: 1–15. doi:10.1093/oxfordjournals.epirev.a036081. PMID 1289108./
- ↑ Willis NJ (August 1997). "Edward Jenner and the eradication of smallpox". Scottish Medical Journal. 42 (4): 118–21. doi:10.1177/003693309704200407. PMID 9507590. S2CID 43179073.
- 1 2 Baxby D (January 1999). "Edward Jenner's Inquiry; a bicentenary analysis". Vaccine. 17 (4): 301–07. doi:10.1016/S0264-410X(98)00207-2. PMID 9987167.
- 1 2 3 Baxby D (2001). Smallpox Vaccine, Ahead of Its Time – How the Late Development of Laboratory Methods and Other Vaccines Affected the Acceptance of Smallpox Vaccine. Berkeley, UK: Jenner Museum. pp. 12–16. ISBN 978-0-9528695-1-1.
- 1 2 3 Baxby D (1981). Jenner's smallpox vaccine; the riddle of vaccinia virus and its origin. London: Heinemann Educational Books. ISBN 0-435-54057-2.
- ↑ Piercey T (August 2002). "Plaque in Memory of Rev. John Clinch". Archived from the original on 20 March 2018. Retrieved 28 May 2014.
- ↑ Handcock G (1996). The Story of Trinity. Trinity: The Trinity Historical Society. p. 1. ISBN 978-098100170-8.
- ↑ "First X, Then Y, Now Z : Landmark Thematic Maps – Medicine". Princeton University Library. 2012. Archived from the original on 13 September 2018. Retrieved 22 May 2018.
- ↑ Morman ET (2006). "Smallpox". In Finkelman P (ed.). Encyclopedia of the New American Nation. Charles Scribner's Sons. pp. 207–08.
- 1 2 Hopkins DR (2002). The greatest killer : smallpox in history, with a new introduction. Chicago: University of Chicago Press. ISBN 978-0-226-35168-1.
- ↑ Bazin H (2000). The Eradication of Smallpox. London: Academic Press. pp. 94–102. ISBN 978-0-12-083475-4.
- ↑ Rusnock A (2009). "Catching cowpox: the early spread of smallpox vaccination, 1798–1810". Bulletin of the History of Medicine. 83 (1): 17–36. doi:10.1353/bhm.0.0160. PMID 19329840. S2CID 24344691.
- ↑ Smith MM (1970). "The 'Real Expedición Marítima de la Vacuna' in New Spain and Guatemala". Trans Amer. Phil. Soc. New Series. 64 (4): 1–74. doi:10.2307/1006158. JSTOR 1006158.
- ↑ "Nova et Vetera". Br Med J. 1 (2370): 1297–1298. 2 June 1906. doi:10.1136/bmj.1.2370.1297. ISSN 0007-1447. PMC 2381502. PMID 20762710. Archived from the original on 17 November 2021. Retrieved 9 April 2022.
- ↑ C. Meyer, S. Reiter (1 December 2004), "Impfgegner und Impfskeptiker", Bundesgesundheitsblatt – Gesundheitsforschung -Gesundheitsschutz (in German), vol. 47, no. 12, pp. 1182–1188, doi:10.1007/s00103-004-0953-x, ISSN 1437-1588, PMID 15583889, S2CID 23282373, archived from the original on 20 May 2022, retrieved 9 April 2022
{{citation}}: CS1 maint: unrecognized language (link) - ↑ Silvia Klein, Irene Schöneberg, Gérard Krause (21 October 2012), "Vom Zwang zur Pockenschutzimpfung zum Nationalen Impfplan", Bundesgesundheitsblatt (in German), vol. 55, pp. 1512–1523, doi:10.25646/1620
{{citation}}: CS1 maint: multiple names: authors list (link) CS1 maint: unrecognized language (link) - ↑ Anders Jarlert: Sveriges Kyrkohistoria. Band 6. Stockholm 2001, S. 33–54.
- ↑ Rudolf Rheinberger: Zum 200. Geburtstag von Landesphysikus Gebhard Schaedler. Archived 27 October 2021 at the Wayback Machine In: Jahrbuch des Historischen Vereins für das Fürstentum Liechtenstein. Band 76. 1976, S. 337–343.
- ↑ Pead PJ (December 2003). "Benjamin Jesty: new light in the dawn of vaccination". Lancet. 362 (9401): 2104–09. doi:10.1016/S0140-6736(03)15111-2. PMID 14697816. S2CID 4254402.
- ↑ Plett PC (2006). "[Peter Plett and other discoverers of cowpox vaccination before Edward Jenner]". Sudhoffs Archiv (in Deutsch). 90 (2): 219–32. JSTOR 20778029. PMID 17338405.
- 1 2 Williams G (2010). Angel of Death; the story of smallpox. Basingstoke: Palgrave Macmillan. pp. 162–73. ISBN 978-0-230-27471-6.
- 1 2 3 Williamson S (2007). The Vaccination Controversy; the rise, reign and decline of compulsory vaccination. Liverpool: Liverpool University Press. ISBN 9781846310867.
- ↑ George NA (November 1952). "Compulsory smallpox vaccination; the University City, Missouri, case". Public Health Reports. 67 (11): 1135–38. doi:10.2307/4588305. JSTOR 4588305. PMC 2030845. PMID 12993980.
- ↑ "Toward a Twenty-First-Century Jacobson v. Massachusetts" (PDF). Harvard Law Review. The Harvard Law Review Association. 121 (7): 1823–1824. May 2008. Archived (PDF) from the original on 26 October 2014. Retrieved 13 March 2014.
- ↑ "A Brief Biography of Dr. Louis T. Wright". North by South: from Charleston to Harlem, the great migration. Archived from the original on 20 October 2017. Retrieved 23 September 2006.
- ↑ "Spotlight on Black Inventors, Scientists, and Engineers". Department of Computer Science of Georgetown University. Archived from the original on 7 September 2006. Retrieved 23 September 2006.
- 1 2 Copeman SM (May 1898). "The Milroy Lectures on the Natural History of Vaccina: Delivered at the Royal College of Physicians". British Medical Journal. 1 (1951): 1312–18. doi:10.1136/bmj.1.1951.1312. PMC 2411485. PMID 20757828.
- 1 2 Didgeon JA (May 1963). "Development of Smallpox Vaccine in England in the Eighteenth and Nineteenth Centuries". British Medical Journal. 1 (5342): 1367–72. doi:10.1136/bmj.1.5342.1367. PMC 2124036. PMID 20789814.
- ↑ Bazin 2000 p. 122.
- ↑ Creighton C (1887). The Natural History of Cowpox and Vaccinal Syphilis. London: Cassell.
- ↑ Copeman SM (1892). "The Bacteriology of Vaccine Lymph". In Shelley CE (ed.). Transactions of the Seventh International Congress of Hygiene and Demography. Eyre and Spottiswoode. pp. 319–26. Retrieved 14 January 2014.
- ↑ Copeman PW (February 1998). "Extinction of the speckled monster celebrated in 1996". Journal of Medical Biography. 6 (1): 39–42. doi:10.1177/096777209800600108. PMID 11619875. S2CID 8918951.
- ↑ Dixon CW (1962). Smallpox. London: J. & A. Churchill. pp. 280–81.
- ↑ Special Commission (1900). "Report of the Lancet Special Commission on Glycerinated Calf Lymph Vaccines". Lancet. 155 (4000): 1227–36. doi:10.1016/s0140-6736(01)96895-3.
- ↑ Collier LH (March 1955). "The development of a stable smallpox vaccine". The Journal of Hygiene. 53 (1): 76–101. doi:10.1017/S002217240000053X. PMC 2217800. PMID 14367805.
- ↑ "Professor Leslie Collier". The Telegraph. 22 March 2011. Archived from the original on 12 January 2022. Retrieved 2 May 2013.
- ↑ Baxby D (October 2005). "Development of a stable smallpox vaccine: Collier L. J Hyg 1955; 53: 76–101". Epidemiology and Infection. 133 (Suppl. 1): S25–27. doi:10.1017/S0950268805004280. PMID 24965243.
- ↑ Kirkup JR (2006). The Evolution of Surgical Instruments. Novato, California: Norman Publishing. pp. 419–37. ISBN 978-0-930405-86-1.
- ↑ Henderson DA (2009). Smallpox; the death of a disease. Amherst, New York: Prometheus Books. pp. 26–27. ISBN 978-1-59102-722-5.
- ↑ Smithson C, Kampman S, Hetman BM, Upton C (2014). "Incongruencies in Vaccinia Virus Phylogenetic Trees". Computation. 2 (4): 182–98. doi:10.3390/computation2040182.
- ↑ Jenner E (1798). An Inquiry into the Causes and Effects of the Variolæ Vaccinæ. London: Self-published.
- 1 2 Esparza J, Schrick L, Damaso CR, Nitsche A (December 2017). "Equination (inoculation of horsepox): An early alternative to vaccination (inoculation of cowpox) and the potential role of horsepox virus in the origin of the smallpox vaccine". Vaccine. 35 (52): 7222–30. doi:10.1016/j.vaccine.2017.11.003. PMID 29137821.
- ↑ Taylor HH (26 October 1889). "What is Vaccinia?". British Medical Journal. 2 (1504): 951–52. ISSN 0007-1447. PMC 2155820.
- ↑ Douglas AJ (October 1915). "An Outbreak of Cowpox". American Journal of Public Health. 5 (10): 1036–37. doi:10.2105/ajph.5.10.1036. PMC 1286723. PMID 18009328.
- ↑ Copeman SM (1899). Vaccination: Its Natural History and Pathology. New York: Macmillan.
- 1 2 Downie AW (April 1939). "The Immunological Relationship of the Virus of Spontaneous Cowpox to Vaccinia Virus". British Journal of Experimental Pathology. 20 (2): 158–76. ISSN 0007-1021. PMC 2065307.
- ↑ Smith GL, Vanderplasschen A (1998). "Extracellular Enveloped Vaccinia virus: Entry, Egress, and Evasion". In Enjuanes L, Siddel SG, Spaan W (eds.). Coronaviruses and Arteriviruses. Vol. 440. Springer Science & Business Media. p. 396. ISBN 978-0-306-45910-8. PMID 9782308.
- ↑ "ICTV Taxonomy history: Cowpox virus". talk.ictvonline.org. 14 April 2021. Archived from the original on 15 April 2021. Retrieved 9 April 2022.
Varidnaviria > Bamfordvirae > Nucleocytoviricota > Pokkesviricetes > Chitovirales > Poxviridae > Chordopoxvirinae > Orthopoxvirus > Cowpox virus
- 1 2 Carroll DS, Emerson GL, Li Y, Sammons S, Olson V, Frace M, et al. (8 August 2011). "Chasing Jenner's vaccine: revisiting cowpox virus classification". PLOS ONE. 6 (8): e23086. Bibcode:2011PLoSO...623086C. doi:10.1371/journal.pone.0023086. PMC 3152555. PMID 21858000.
- ↑ Schrick L, Tausch SH, Dabrowski PW, Damaso CR, Esparza J, Nitsche A (October 2017). "An Early American Smallpox Vaccine Based on Horsepox". The New England Journal of Medicine. 377 (15): 1491–92. doi:10.1056/NEJMc1707600. PMID 29020595.
- ↑ Esparza J (September 2013). "Has horsepox become extinct?". The Veterinary Record. 173 (11): 272–73. doi:10.1136/vr.f5587. PMID 24057497. S2CID 36975171.
- ↑ Noyce RS, Lederman S, Evans DH (19 January 2018). "Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments". PLOS ONE. 13 (1): e0188453. Bibcode:2018PLoSO..1388453N. doi:10.1371/journal.pone.0188453. PMC 5774680. PMID 29351298.
- ↑ Kupferschmidt K (January 2018). "Critics see only risks, no benefits in horsepox paper". Science. 359 (6374): 375–76. Bibcode:2018Sci...359..375K. doi:10.1126/science.359.6374.375. PMID 29371445.
- ↑ Baxby D (1981). Jenner's Smallpox Vaccine: The Riddle of Vaccinia Virus and Its Origin. Heinemann Educational Books. ISBN 978-0-435-54057-9.
- ↑ Operational framework for the deployment of the World Health Organization Smallpox Vaccine Emergency Stockpile in response to a smallpox event. World Health Organization (WHO). 2017. ISBN 978-92-4-151341-8.
- 1 2 3 4 Kneip, Ansbert (26 January 2003). "Pocken-Fieber". Der Spiegel (in Deutsch). Archived from the original on 4 July 2022. Retrieved 4 July 2022.
- ↑ StreetJournal, Sarah LueckStaff Reporter of The Wall (1 April 2002). "Aventis to Donate Smallpox Vaccine To the U.S. Government's Stockpile". Wall Street Journal. Archived from the original on 12 January 2018. Retrieved 4 July 2022.
- ↑ Emergent BioSolutions (3 September 2019). "Emergent BioSolutions Awarded 10-Year HHS Contract to Deliver ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live) Into the Strategic" (Press release). Archived from the original on 8 July 2022. Retrieved 4 July 2022.
- ↑ Bavarian Nordic. "Bavarian Nordic Announces U.S. FDA Approval of Jynneos (Smallpox and Monkeypox Vaccine, Live, Non-Replicating) for Prevention of Smallpox and Monkeypox Disease in Adults" (Press release). Archived from the original on 28 June 2022. Retrieved 4 July 2022.
- 1 2 3 U.S. Department of Health and Human Services (1 July 2022). "HHS Orders 2.5 Million More Doses of JYNNEOS Vaccine For Monkeypox Preparedness" (Press release). Archived from the original on 4 July 2022. Retrieved 4 July 2022.
- ↑ "Clinical Guidance for Smallpox Vaccine Use in a Postevent Vaccination Program". Morbidity and Mortality Weekly Report (64): 1–26. 20 February 2015. Archived from the original on 26 June 2022. Retrieved 4 July 2022.
- 1 2 3 Talbot, TR; Stapleton, JT; Brady, RC; Winokur, PL; Bernstein, DI; Germanson, T; Yoder, SM; Rock, MT; Crowe JE, Jr; Edwards, KM (8 September 2004). "Vaccination success rate and reaction profile with diluted and undiluted smallpox vaccine: a randomized controlled trial". JAMA. 292 (10): 1205–12. doi:10.1001/jama.292.10.1205. PMID 15353533.
- ↑ Costa, Alejandro (5–7 November 2013). "Smallpox vaccine stockpile" (PDF). World Health Organization (WHO). Archived (PDF) from the original on 4 July 2022. Retrieved 4 July 2022.
- ↑ "Smallpox eradication: destruction of variola virus stocks" (PDF). 4 April 2019. Archived (PDF) from the original on 16 May 2022. Retrieved 4 July 2022.
- ↑ "Plan national de réponse à une menace de variole" (PDF). Ministère de la Santé et des Solidarités (France). August 2006. Archived (PDF) from the original on 19 May 2022. Retrieved 4 July 2022.
- ↑ "Vaiolo delle scimmie, 'abbiamo 5 milioni dosi di vaccino: pronti se servirà'" (in Italian). adnkronos. 27 May 2022. Archived from the original on 14 July 2022. Retrieved 14 July 2022.
{{cite web}}: CS1 maint: unrecognized language (link) - ↑ Kenner, J; Cameron, F; Empig, C; Jobes, DV; Gurwith, M (17 November 2006). "LC16m8: an attenuated smallpox vaccine". Vaccine. 24 (47–48): 7009–22. doi:10.1016/j.vaccine.2006.03.087. PMC 7115618. PMID 17052815.
- ↑ Jihyun Kim (23 May 2022). "15개국 퍼진 원숭이 두창, 불안 확산…백신·치료제는?". Newsis. Seoul. Archived from the original on 23 May 2022. Retrieved 23 May 2022.
- ↑ Oh, MD; Lee, JK (July 2012). "Milestones in history of adult vaccination in Korea". Clinical and Experimental Vaccine Research. 1 (1): 9–17. doi:10.7774/cevr.2012.1.1.9. PMC 3623517. PMID 23596574.
- ↑ "Canada orders 65,700 doses of IMVAMUNE smallpox vaccine". Outbreak News Today. 27 August 2014. Archived from the original on 23 July 2021. Retrieved 2 August 2022.
- ↑ "Canada orders more IMVAMUNE smallpox vaccine". Outbreak News Today. 22 March 2021. Archived from the original on 6 June 2022. Retrieved 2 August 2022.
- ↑ "Limited vaccine supply could thwart Canada's efforts to contain monkeypox". CBC. Archived from the original on 1 August 2022. Retrieved 2 August 2022.
- ↑ "News | Bavarian Nordic". www.bavarian-nordic.com. Archived from the original on 26 July 2022. Retrieved 2 August 2022.
- ↑ Government of Canada, Public Services and Procurement Canada (21 April 2022). "Third Generation Smallpox Vaccine (6D024-215700/A)". buyandsell.gc.ca. Archived from the original on 20 June 2022. Retrieved 2 August 2022.
- ↑ Government of Canada, Public Services and Procurement Canada (24 December 2020). "2nd Generation Smallpox Vaccine (6D024-203934/A)". buyandsell.gc.ca. Archived from the original on 8 November 2021. Retrieved 2 August 2022.
Further reading
- Metzger W, Mordmueller BG (July 2007). Metzger W (ed.). "Vaccines for preventing smallpox". The Cochrane Database of Systematic Reviews (3): CD004913. doi:10.1002/14651858.CD004913.pub2. PMC 6532594. PMID 17636779.
External links
| Identifiers: |
|---|
- . Encyclopædia Britannica. Vol. 27 (11th ed.). 1911. pp. 831–834.
- Smallpox Vaccine: Contraindications, Administration, and Adverse Reactions Archived 20 July 2008 at the Wayback Machine
- CDC Smallpox Home Archived 18 March 2021 at the Wayback Machine
- Quick Guide to Preexposure Smallpox Vaccination Archived 23 May 2013 at the Wayback Machine
- Early smallpox Vaccination Certificate Norway 1821 Archived 15 May 2021 at the Wayback Machine GG Archives. Norwegian and English Text
- Episode 1 (of 4): Vaccines Archived 15 August 2021 at the Wayback Machine of the PBS and BBC Four show: Extra Life: A Short History of Living Longer Archived 14 August 2021 at the Wayback Machine