Yellow fever vaccine
.jpg.webp) | |
| Vaccine description | |
|---|---|
| Target disease | Yellow fever |
| Type | Attenuated virus |
| Names | |
| Trade names | YF-Vax, Stamaril |
| Other names | 17D vaccine |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | Subcutaneous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607030 |
| Legal | |
| Legal status | |
Yellow fever vaccine is a vaccine that protects against yellow fever.[4] Yellow fever is a viral infection that occurs in Africa and South America.[4] Most people begin to develop immunity within ten days and 99 percent are protected within one month of vaccination, and this appears to be lifelong.[4] The vaccine can be used to control outbreaks of disease.[4] It is given either by injection into a muscle or just under the skin.[4]
The World Health Organization (WHO) recommends routine immunization in all countries where the disease is common.[4] This should typically occur between nine and twelve months of age.[4] Those traveling to areas where the disease occurs should also be immunized.[4] Additional doses after the first are generally not needed.[5]
Yellow fever vaccine is generally safe.[4] This includes in those with HIV infection but without symptoms.[4] Mild side effects may include headache, muscle pains, pain at the injection site, fever, and rash.[4] Severe allergies occur in about eight per million doses, serious neurological problems occur in about four per million doses, and organ failure occurs in about three per million doses.[4] It appear to be safe in pregnancy and therefore recommended among those who will be potentially exposed.[4] It should not be given to those with very poor immune function.[6]
Yellow fever vaccine came into use in 1938.[7] It is on the World Health Organization's List of Essential Medicines.[8] The vaccine is made from weakened yellow fever virus.[4] Some countries require a yellow fever vaccination certificate before entry from a country where the disease is common.[4] The wholesale price in the developing world is between US$4.30 and US$21.30 per dose as of 2014.[9] In the United States it costs between US$50 and US$100.[10] The vaccine is made from weakened yellow fever virus.[4] In Canada it costs about $180 CAD as of 2023.[11] Some countries require a yellow fever vaccination certificate before entry from a country where the disease is common.[4]
Medical uses

People most at risk of contracting the virus should be vaccinated. Woodcutters working in tropical areas should be particularly targeted for vaccination. Insecticides, protective clothing, and screening of houses are helpful, but not always sufficient for mosquito control; people should always use an insecticide spray while in certain areas. In affected areas, mosquito control methods have proven effective in decreasing the number of cases.[12]
Travelers should have the vaccine ten days prior to being in an endemic area.
On 17 May 2013, the World Health Organization (WHO) Strategic Advisory Group of Experts on immunization (SAGE) announced that a 'booster' dose of yellow fever (YF) vaccine, ten years after a primary dose, is not necessary. Since yellow fever vaccination began in the 1930s, only 12 known cases of yellow fever post-vaccination have been identified, after 600 million doses have been dispensed. Evidence showed that among this small number of "vaccine failures", all cases developed the disease within five years of vaccination. This demonstrates that immunity does not decrease with time.[13]
Schedule
The World Health Organization recommends the vaccine between the ages of 9 and 12 months in areas where the disease is common.[4] Anyone over the age of nine months who has not been previously immunized and either lives in or is traveling to an area where the disease occurs should also be immunized.[4]
Dosage
The defined daily dose is not established.[14]
Side effects
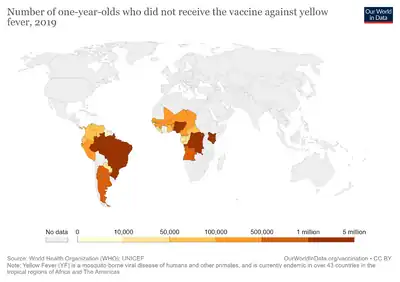
The yellow fever 17D vaccine is considered safe, with over 500 million doses given and very few documented cases of vaccine associated illness (62 confirmed cases and 35 deaths as of January 2019).[15] In no case of vaccine-related illness has there been evidence of the virus reverting to a virulent phenotype.
The majority of adverse reactions to the 17D vaccine result from allergic reaction to the eggs in which the vaccine is grown. Persons with known egg allergy should discuss this with their physician prior to vaccination. In addition, there is a small risk of neurologic disease and encephalitis, particularly in individuals with compromised immune systems and very young children. The 17D vaccine is contraindicated in (among others) infants between zero and six months or over 59 years of age,[16] people with thymus disorders associated with abnormal immune cell function, people with primary immunodeficiencies, and anyone with a diminished immune capacity including those taking immunosuppressant drugs.[17]
There is a small risk of more severe yellow fever-like disease associated with the vaccine. This reaction, known as yellow fever vaccine-associated acute viscerotropic disease (YEL-AVD),[18] causes a fairly severe disease closely resembling yellow fever caused by virulent strains of the virus. The risk factors for YEL-AVD are not known, although it has been suggested that it may be genetic. The 2'-5'-oligoadenylate synthase (OAS) component of the innate immune response has been shown to be particularly important in protection from Flavivirus infection. Another reaction to the yellow fever vaccine is known as yellow fever vaccine-associated acute neurotropic disease (YEL-AND).
The Canadian Medical Association published a 2001 CMAJ article entitled "Yellow fever vaccination: be sure the patient needs it".[19] The article begins by stating that of the seven people who developed system failure within two to five days of the vaccine in 1996–2001, six died "including 2 who were vaccinated even though they were planning to travel to countries where yellow fever has never been reported." The article cites that "3 demonstrated histopatholic changes consistent with wild yellow fever virus." The author recommends vaccination for only non-contraindicated travelers (see the articles list) and those travelers going where yellow fever activity is reported or in the endemic zone which can be found mapped at the CDC website cited below. In addition, the 2010 online edition of the Center for Disease Control Traveler's Health Yellow Book that between 1970 and 2002 only "nine cases of yellow fever were reported in unvaccinated travelers from the United States and Europe who traveled" to West Africa and South America, and 8 of the 9 died. However, it goes on to cite "only 1 documented case of yellow fever in a vaccinated traveler. This nonfatal case occurred in a traveler from Spain who visited several West African countries in 1988".[20]
History
The first attempts to develop a yellow fever vaccine followed the opening of the Panama Canal in 1912, which increased global exposure to the disease.[21] The Japanese bacteriologist Hideyo Noguchi led investigations for the Rockefeller Foundation in Ecuador that resulted in a vaccine based on his theory that the disease was caused by a leptospiral bacterium. However, other investigators could not duplicate his results and the ineffective vaccine was eventually abandoned.
Another vaccine was developed from the "French strain" of the virus, obtained by Pasteur Institute scientists from a man in Dakar, Senegal, who survived his bout with the disease. This vaccine could be administered by scarification, like the smallpox vaccine, and was given in combination to produce immunity to both diseases, but it also had severe systemic and neurologic complications in a few cases. Attempts to attenuate the virus used in the vaccine failed. Scientists at the Rockefeller Foundation developed another vaccine derived from the serum of an African named Asibi in 1927, the first isolation of the virus from a human.[22] It was safer but involved the use of large amounts of human serum, which limited widespread use. Both vaccines were in use for several years, the Rockefeller vaccine in the Western hemisphere and England, and the Pasteur Institute vaccine in France and its African colonies.
In 1937, Max Theiler, working with Hugh Smith at the Rockefeller Foundation to improve the vaccine from the "Asibi" strain, discovered that a favorable chance mutation in the attenuated virus had produced a highly effective strain that was named 17D. After field trials in Brazil, over one million people were vaccinated by 1939, without severe complications.[7] For his work on the yellow fever vaccine, Theiler received the 1951 Nobel Prize in Physiology or Medicine.[23] Only the 17D vaccine remains in use today.[4]
Two vaccines were developed in the 1930s, after scientists isolated yellow fever virus in West Africa.[24] The vaccine 17D was developed by the South African microbiologist Max Theiler at the Rockefeller Institute in New York City. This vaccine was widely used by the U.S. Army during World War II.[25] Following the work of Ernest Goodpasture, Theiler used chicken eggs to culture the virus and won a Nobel Prize in 1951 for this achievement. A French team developed the French neurotropic vaccine (FNV), which was extracted from mouse brain tissue. Since this vaccine was associated with a higher incidence of encephalitis, FNV was not recommended after 1961. Vaccine 17D is still in use, and more than 400 million doses have been distributed. Little research has been done to develop new vaccines. Some researchers worry that the 60-year-old technology for vaccine production may be too slow to stop a major new yellow-fever epidemic. Newer vaccines, based on vero cells, are in development and should replace 17D at some point.[26][27][28]
Availability
The outbreak of yellow fever in Angola in January 2016 has raised concerns about whether the global supply of vaccine is adequate to meet the need during a large epidemic or pandemic of the disease.[29] Routine childhood immunization has been suspended in other African countries to ensure an adequate supply in the vaccination campaign against the outbreak in Angola.[30] Emergency stockpiles of vaccine diverted to Angola, which consisted of about 10 million doses at the end of March 2016, had become exhausted,[31][32] but were being replenished by May 2016.[33] However, in August it was reported that about one million doses of six million shipped in February had been sent to the wrong place or not kept cold enough to ensure efficacy, resulting in shortages to fight the spreading epidemic in DR Congo.[34] As an emergency measure, experts have suggested using a fractional dose (1/5 or 1/10 of the usual dose) to extend existing supplies of vaccine.[35][30] Others have noted that switching manufacturing processes to modern cell-culture technology might improve vaccine supply shortfalls.[36] Manufacture of the current vaccine is slow and laborious. A new vaccine under investigation is made by a different means.[37] On June 17, the WHO agreed to the use of 1/5 the usual dose as an emergency measure during the ongoing outbreak in Angola and the DR Congo.[38] The fractional dose would not qualify for a yellow fever certificate of vaccination for travelers.
Increases in cases of yellow fever in endemic areas of Africa and South America in the 1980s were addressed by the WHO Yellow Fever Initiative launched in the mid-2000s.[39] The initiative was supported by the Gavi Alliance, a collaboration of the WHO, UNICEF, vaccine manufacturers, and private philanthropists such as the Bill & Melinda Gates Foundation. Gavi-supported vaccination campaigns since 2011 have covered 88 million people in 14 countries considered at "high-risk" of a yellow fever outbreak (Angola was considered "medium risk"). Activities of the Yellow Fever Initiative are managed through an international coordinating group established in 2000 when a global shortage of yellow fever vaccine had developed because of the long lead time to manufacture the vaccine.[40] As of 2013, there were four WHO-qualified manufacturers: Bio-Manguinhos in Brazil (with the Oswaldo Cruz Foundation), Institute Pasteur in Dakar, Senegal, the Federal State Unitary Enterprise of Chumakov Institute in Russia, and Sanofi Pasteur, the French pharmaceutical company.[31][41] Two other manufacturers supply domestic markets: Wuhan Institute of Biological Products in China and Sanofi Pasteur in the United States.[36] Seth Berkley, the chief executive of Gavi, has warned about the worldwide threat of yellow fever because of shortages in vaccine supplies.[42]
Demand for yellow vaccine for preventive campaigns has increased from about five million doses per year to a projected 62 million per year by 2014.[43] UNICEF reported in 2013 that supplies were insufficient. Manufacturers are producing about 35 million of the 64 million doses needed per year.[44]
Demand for the yellow fever vaccine has continued to increase due to the growing number of countries implementing yellow fever vaccination as part of their routine immunization programmes.[45] Upsurges in yellow fever outbreaks in Angola (2015), Democratic Republic of Congo (2016), Uganda (2016), and in Nigeria and Brazil in 2017 have further increased demand, while straining global vaccine supply.[45][46] Therefore, to vaccinate susceptible populations in preventive mass immunization campaigns during outbreaks. As of 2016, fractional dosing of the vaccine is considered as a dose-sparing strategy to maximize limited vaccine supplies.[45] Fractional dose vaccination refers to administration of a reduced volume of vaccine dose, which has been reconstituted as per manufacturer recommendations.[45][35][47] The first practical use of fractional doses of the yellow fever vaccine was in response to the 2016 outbreak in the Democratic Republic of Congo.[45]
References
- 1 2 Use During Pregnancy and Breastfeeding
- ↑ "Stamaril powder and solvent for suspension for injection in pre-filled syringe - Summary of Product Characteristics (SmPC)". (emc). 9 September 2019. Archived from the original on 29 December 2019. Retrieved 28 December 2019.
- ↑ "YF-Vax". U.S. Food and Drug Administration (FDA). 6 August 2019. Archived from the original on 19 October 2020. Retrieved 28 December 2019.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 World Health Organization (July 2013). "Vaccines and vaccination against yellow fever : WHO Position Paper — June 2013". Weekly Epidemiological Record. 88 (27): 269–83. hdl:10665/242089. PMID 23909008.
- ↑ Staples JE, Bocchini JA, Rubin L, Fischer M, Centers for Disease Control (CDC) (19 June 2015). "Yellow Fever Vaccine Booster Doses: Recommendations of the Advisory Committee on Immunization Practices, 2015". MMWR Morb. Mortal. Wkly. Rep. 64 (23): 647–50. PMC 4584737. PMID 26086636.
- ↑ "Yellow Fever Vaccine". Centers for Disease Control and Prevention (CDC). 13 December 2011. Archived from the original on 9 December 2015. Retrieved 15 December 2015.
- 1 2 Norrby E (November 2007). "Yellow fever and Max Theiler: the only Nobel Prize for a virus vaccine". J. Exp. Med. 204 (12): 2779–84. doi:10.1084/jem.20072290. PMC 2118520. PMID 18039952.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Vaccine, Yellow Fever". International Drug Price Indicator Guide. Archived from the original on 24 December 2018. Retrieved 6 December 2015.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 318. ISBN 9781284057560.
- ↑ "Travel Clinic Fees". Nova Travel Clinic. Archived from the original on 2 June 2023. Retrieved 10 November 2023.
- ↑ "Joint Statement on Mosquito Control in the United States from the U.S. Environmental Protection Agency (EPA) and the U.S. Centers for Disease Control and Prevention (CDC)" (PDF). Environmental Protection Agency. 3 May 2000. Archived from the original on 10 October 2006. Retrieved 25 June 2006.
- ↑ "Vaccines" (Press release). World Health Organization (WHO). Archived from the original on 9 June 2013.
- ↑ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 24 September 2020. Retrieved 22 September 2020.
- ↑ "What are the risks of dying from having the yellow fever vaccine?". 11 January 2019. Archived from the original on 12 October 2019. Retrieved 12 October 2019.
- ↑ "Yellow Fever Vaccine Information Statement". Centers for Disease Control and Prevention (CDC). 30 March 2011. Archived from the original on 21 September 2013.
- ↑ "Yellow Fever". Centers for Disease Control and Prevention (CDC). Archived from the original on 16 January 2013.
- ↑ Bae HG, Domingo C, Tenorio A, et al. (June 2008). "Immune response during adverse events after 17D-derived yellow fever vaccination in Europe". J. Infect. Dis. 197 (11): 1577–84. doi:10.1086/587844. PMID 18419548.
- ↑ Weir E (October 2001). "Yellow fever vaccination: be sure the patient needs it". CMAJ : Canadian Medical Association Journal. 165 (7): 941. PMC 81520. PMID 11599337.
- ↑ Gershman, Mark; Schroeder, Betsy; Staples, J. Erin. "Yellow Fever". Yellow Book. Center for Disease Control (Canada). Archived from the original on 1 July 2011. Retrieved 1 July 2011.
- ↑ Frierson, J. Gordon (1 June 2010). "The Yellow Fever Vaccine: A History". The Yale Journal of Biology and Medicine. 83 (2): 77–85. ISSN 0044-0086. PMC 2892770. PMID 20589188.
- ↑ Stokes A, Bauer JH, Hudson NP (1928). "Experimental transmission of yellow fever to laboratory animals". Am J Trop Med Hyg. 8 (2): 103–104. doi:10.4269/ajtmh.1928.s1-8.103.
- ↑ "Max Theiler – Biography". Archived from the original on 20 January 2009. Retrieved 15 January 2009.
- ↑ Bigon L (2014). "Transnational Networks of Administrating Disease and Urban Planning in West Africa: The Inter-Colonial Conference on Yellow Fever, Dakar, 1928". GeoJournal. 79 (1): 103–111. doi:10.1007/s10708-013-9476-z.
- ↑ McNeill JR (1 April 2004). "Yellow Jack and Geopolitics: Environment, Epidemics, and the Struggles for Empire in the American Tropics, 1650–1825". OAH Magazine of History. 18 (3): 9–13. doi:10.1093/maghis/18.3.9. Archived from the original on 20 December 2016.
- ↑ Tolle MA (April 2009). "Mosquito-borne diseases". Curr Probl Pediatr Adolesc Health Care. 39 (4): 97–140. doi:10.1016/j.cppeds.2009.01.001. PMID 19327647.
- ↑ National Institutes of Health (27 July 2016). "NIH launches early-stage yellow fever vaccine trial" (Press release). United States Department of Health and Human Services. Archived from the original on 26 August 2016. Retrieved 14 July 2019.
- ↑ National Institute of Allergy and Infectious Diseases (NIAID) (1 June 2018), A Phase I Trial to Evaluate the Safety, Reactogenicity, and Immunogenicity of MVA-BN Yellow Fever Vaccine With and Without Montanide ISA-720 Adjuvant in 18-45 Year Old Healthy Volunteers (NCT number: NCT02743455), United States National Library of Medicine, archived from the original on 12 May 2019, retrieved 14 July 2019.
- ↑ "Yellow Fever Deaths Reach 250 in Angola | HealthMap". www.healthmap.org. Archived from the original on 29 April 2016. Retrieved 28 April 2016.
- 1 2 Monath TP, Woodall JP, Gubler DJ, Yuill TM, Mackenzie JS, Martins RM, Reiter P, Heymann DL (April 2016). "Yellow fever vaccine supply: a possible solution". Lancet. 387 (10028): 1599–600. doi:10.1016/S0140-6736(16)30195-7. PMID 27116054.
- 1 2 Kupferschmidt, Kai (4 April 2016). "Angolan yellow fever outbreak highlights dangerous vaccine shortage". Science. Archived from the original on 25 April 2016. Retrieved 24 April 2016.
- ↑ "Angola extends yellow fever vaccination campaign to Huambo and Benguela provinces". World Health Organization (WHO) (Press release). Archived from the original on 23 April 2016. Retrieved 24 April 2016.
- ↑ Kupferschmidt, Kai (19 May 2016). "Yellow fever threat is 'serious' but not an 'emergency,' WHO says". Science. doi:10.1126/science.aaf5736. ISSN 0036-8075.
- ↑ "UN bungles response to Africa's yellow fever outbreak". Archived from the original on 6 August 2016. Retrieved 5 August 2016.
- 1 2 World Health Organization (June 2017). "Yellow fever vaccine: WHO position on the use of fractional doses – June 2017". Weekly Epidemiological Record. 92 (25): 345–50. hdl:10665/255754. PMID 28643507.
- 1 2 Barrett AD (July 2016). "Yellow Fever in Angola and Beyond--The Problem of Vaccine Supply and Demand". The New England Journal of Medicine. 375 (4): 301–3. doi:10.1056/NEJMp1606997. PMID 27276108. Archived from the original on 27 August 2021. Retrieved 5 December 2019.
- ↑ "NIH launches early-stage yellow fever vaccine trial". 26 July 2016. Archived from the original on 26 August 2016. Retrieved 15 August 2016.
- ↑ "Lower doses of yellow fever vaccine could be used in emergencies". World Health Organization (WHO) (Press release). Archived from the original on 18 June 2016. Retrieved 19 June 2016.
- ↑ "The Yellow fever initiative: an introduction". World Health Organization (WHO). Archived from the original on 10 May 2016. Retrieved 23 April 2016.
- ↑ "Yellow fever vaccine: a global partnership". World Health Organization (WHO). Archived from the original on 31 March 2016. Retrieved 23 April 2016.
- ↑ "Yellow Fever Vaccine: Current Outlook" (PDF). Unicef. Archived (PDF) from the original on 4 March 2016. Retrieved 23 April 2016.
- ↑ Berkley, Seth (15 May 2017). "The Looming Threat of Yellow Fever". The New York Times. Archived from the original on 8 September 2017. Retrieved 17 May 2017.
- ↑ "Fever Vaccine: Current Outlook November 2013" (PDF). UNICEF. Archived (PDF) from the original on 4 March 2016. Retrieved 23 April 2016.
- ↑ "Complacency Led to Resurgence of Yellow Fever". www.globalhealthnow.org. Archived from the original on 7 May 2016. Retrieved 23 April 2016.
- 1 2 3 4 5 World Health Organization (July 2016). Fractional dose yellow fever vaccine as a dose-sparing option for outbreak response: WHO Secretariat information paper. hdl:10665/246236. WHO/YF/SAGE/16.1.
- ↑ "WHO supports the immunization of 874 000 people against yellow fever in Nigeria" (Press release). World Health Organization (WHO). 16 October 2017. Archived from the original on 18 April 2018. Retrieved 2 September 2018.
- ↑ World Health Organization (October 2017). "WHO position on the use of fractional doses - June 2017, addendum to vaccines and vaccination against yellow fever WHO: Position paper - June 2013". Vaccine. 35 (43): 5751–5752. doi:10.1016/j.vaccine.2017.06.087. PMID 28689653.
External links
| Identifiers: |
|---|
- Yellow Fever Vaccine at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Yellow Fever Vaccine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 28 October 2020. Retrieved 18 May 2020.