RTS,S
| Vaccine description | |
|---|---|
| Target disease | P. falciparum; to a lesser extent Hepatitis B |
| Type | Protein subunit |
| Names | |
| Trade names | Mosquirix |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | intramuscular injection (0.5 mL)[1] |
| Legal | |
| Legal status |
|
RTS,S/AS01 (trade name Mosquirix) is a recombinant protein-based malaria vaccine. It is the only malaria vaccine approved and in wide use. As of April 2022, the vaccine has been given to 1 million children living in areas with moderate-to-high malaria transmission, with millions more doses to be provided as the vaccine's production expands.[2][3] It requires at least three doses in infants by age 2, with a fourth dose extending the protection for another 1-2 years.[2] The vaccine reduces hospital admissions from severe malaria by around 30%.[2]
Medical uses

RTS,S/AS01 (commercial name Mosquirix) is the only malaria vaccine approved and in current use. The vaccine's use requires at least three doses in infants by age 2, with a fourth dose extending the protection for another 1-2 years.[2]
The vaccine reduces hospital admissions from severe malaria by around 30%.[2]
History
Potential malaria vaccines have been an intense area of research since the 1960s.[5] SPf66 was tested extensively in endemic areas in the 1990s, but clinical trials showed it to be insufficiently effective.[6] Other vaccine candidates, targeting the blood-stage of the malaria parasite's life cycle, had also been insufficient on their own.[7]
The RTS,S vaccine was conceived of and created in the late 1980s by scientists working at SmithKline Beecham Biologicals (now GlaxoSmithKline (GSK) Vaccines) laboratories in Belgium.[8] The vaccine was further developed through a collaboration between GSK and the Walter Reed Army Institute of Research in the U.S. state of Maryland[9] and has been funded in part by the non-profit PATH Malaria Vaccine Initiative (MVI) and the Bill and Melinda Gates Foundation.
RTS,S was engineered using genes from the outer protein of P. falciparum malaria parasite and a portion of a hepatitis B virus plus a chemical adjuvant to boost the immune response. Infection is prevented by inducing high antibody titers that block the parasite from infecting the liver.[10] In November 2012, a phase III trial of RTS,S found that it provided modest protection against both clinical and severe malaria in young infants.[11] The RTS,S-based vaccine formulation had previously been demonstrated to be safe, well tolerated, immunogenic, and to potentially confer partial efficacy in both malaria-naive and malaria-experienced adults as well as children.[12]
In October 2013, preliminary results of a phase III clinical trial indicated that RTS,S/AS01 reduced the number of cases among young children by almost 50 percent and among infants by around 25 percent. The study ended in 2014. The effects of a booster dose were positive, even though overall efficacy seems to wane with time. After four years, reductions were 36 percent for children who received three shots and a booster dose. Missing the booster dose reduced the efficacy against severe malaria to a negligible effect. The vaccine was shown to be less effective for infants. Three doses of vaccine plus a booster reduced the risk of clinical episodes by 26 percent over three years but offered no significant protection against severe malaria.[13]
In a bid to accommodate a larger group and guarantee a sustained availability for the general public, GSK applied for a marketing license with the European Medicines Agency (EMA) in July 2014.[14] GSK treated the project as a non-profit initiative, with most funding coming from the Gates Foundation, a major contributor to malaria eradication.[15]
In July 2015, Mosquirix received a positive scientific opinion from the European Medicines Agency on the proposal for the vaccine to be used to vaccinate children aged 6 weeks to 17 months outside the European Union.[1] It is the world's first licensed malaria vaccine and also the first vaccine licensed for use against a human parasitic disease of any kind.[16] On 23 October 2015, WHO's Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Committee (MPAC) jointly recommended a pilot implementation of the vaccine in Africa.[17]
In November 2016, WHO announced that the RTS,S vaccine would be rolled out in pilot projects in three countries in sub-Saharan Africa. This pilot project for vaccination was launched on 23 April 2019 in Malawi, on 30 April 2019 in Ghana, and on 13 September 2019 in Kenya.[18][19]
In October 2021, the vaccine was endorsed by the World Health Organization (WHO) for "broad use" in children, making it the first malaria vaccine candidate, and first vaccine to address parasitic infection, to receive this recommendation.[20][21][22] As of April 2022, 1 million children in Ghana, Kenya and Malawi have received at least one shot of the vaccine.[2]
In August 2022, UNICEF awarded a contract to GSK to purchase 18 million doses of the RTS,S vaccine over three years. More than 30 countries have areas with moderate to high malaria transmission where the vaccine is expected to be useful.[3]
Components and mechanism
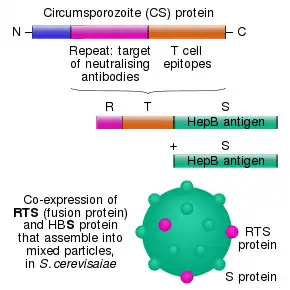
The RTS,S vaccine is based on a protein construct first developed by GlaxoSmithKline in 1986. It was named RTS because it was engineered using genes from the repeat ('R') and T-cell epitope ('T') of the pre-erythrocytic circumsporozoite protein (CSP) of the Plasmodium falciparum malaria parasite together with a viral surface antigen ('S') of the hepatitis B virus (HBsAg).[9] This protein was then mixed with additional HBsAg to improve purification, hence the extra "S".[9] Together, these two protein components assemble into soluble virus-like particles similar to the outer shell of a hepatitis B virus.[23]
A chemical adjuvant (AS01, specifically AS01E) was added to increase the immune system response.[24] Infection is prevented by inducing humoral and cellular immunity, with high antibody titers, that block the parasite from infecting the liver.[25]
The T-cell epitope of CSP is O-fucosylated in Plasmodium falciparum[26][27] and Plasmodium vivax,[28] while the RTS,S vaccine produced in yeast is not.[29]
References
- 1 2 "Mosquirix H-W-2300". European Medicines Agency (EMA). Archived from the original on 23 November 2019. Retrieved 4 March 2021.
- 1 2 3 4 5 6 "First malaria vaccine hits 1 million dose milestone — although it has its shortcomings". NPR. 13 May 2022. Archived from the original on 13 November 2022. Retrieved 2 January 2023.
- 1 2 "Millions more children to benefit from malaria vaccine as UNICEF secures supply". UNICEF. 16 August 2022. Archived from the original on 2 January 2023. Retrieved 2 January 2023.
- ↑ "RTS,S Malaria Vaccine: 2019 Partnership Award Honoree". YouTube. Global Health Technologies Coalition. Retrieved 6 October 2021.
- ↑ Hill AV (October 2011). "Vaccines against malaria". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 366 (1579): 2806–14. doi:10.1098/rstb.2011.0091. PMC 3146776. PMID 21893544.
- ↑ Graves P, Gelband H (April 2006). Graves PM (ed.). "Vaccines for preventing malaria (SPf66)". The Cochrane Database of Systematic Reviews (2): CD005966. doi:10.1002/14651858.CD005966. PMC 6532709. PMID 16625647.
- ↑ Graves P, Gelband H (October 2006). Graves PM (ed.). "Vaccines for preventing malaria (blood-stage)". The Cochrane Database of Systematic Reviews (4): CD006199. doi:10.1002/14651858.CD006199. PMC 6532641. PMID 17054281.
- ↑ "HYBRID PROTEIN BETWEEN CS FROM PLASMODIUM AND HBsAG". Archived from the original on 20 October 2016. Retrieved 29 January 2023.
- 1 2 3 Heppner DG, Kester KE, Ockenhouse CF, Tornieporth N, Ofori O, Lyon JA, et al. (March 2005). "Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research". Vaccine. 23 (17–18): 2243–50. doi:10.1016/j.vaccine.2005.01.142. PMID 15755604. S2CID 22824901. Archived from the original on 23 July 2018.
- ↑ Foquet L, Hermsen CC, van Gemert GJ, Van Braeckel E, Weening KE, Sauerwein R, et al. (January 2014). "Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection". The Journal of Clinical Investigation. 124 (1): 140–4. doi:10.1172/JCI70349. PMC 3871238. PMID 24292709.
- ↑ Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, et al. (December 2012). "A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants". The New England Journal of Medicine. 367 (24): 2284–95. doi:10.1056/NEJMoa1208394. PMID 23136909.
- ↑ Regules JA, Cummings JF, Ockenhouse CF (May 2011). "The RTS,S vaccine candidate for malaria". Expert Review of Vaccines. 10 (5): 589–99. doi:10.1586/erv.11.57. PMID 21604980. S2CID 20443829. Archived from the original on 7 October 2021. Retrieved 29 January 2023.
- ↑ Borghino D (27 April 2015). "Malaria vaccine candidate shown to prevent thousands of cases". www.gizmag.com. Archived from the original on 6 May 2016. Retrieved 11 June 2016.
- ↑ "GSK announces EU regulatory submission of malaria vaccine candidate RTS,S" (Press release). GSK. 24 July 2014. Archived from the original on 4 December 2016. Retrieved 30 July 2015.
- ↑ Kelland K (7 October 2013). "GSK aims to market world's first malaria vaccine". Reuters. Archived from the original on 18 January 2016. Retrieved 9 December 2013.
- ↑ Walsh F (24 July 2015). "Malaria vaccine gets 'green light'". BBC News. Archived from the original on 21 July 2020. Retrieved 25 July 2015.
- ↑ Stewart S (23 October 2015). "Pilot implementation of first malaria vaccine recommended by WHO advisory groups" (Press release). Geneva: World Health Organization. Archived from the original on 19 September 2021.
- ↑ Alonso P (19 June 2019). "Letter to partners – June 2019" (Press release). Wuxi: World Health Organization. Archived from the original on 31 January 2022. Retrieved 22 October 2019.
- ↑ "Malaria vaccine launched in Kenya: Kenya joins Ghana and Malawi to roll out landmark vaccine in pilot introduction" (Press release). Homa Bay: World Health Organization. 13 September 2019. Archived from the original on 22 October 2019. Retrieved 22 October 2019.
- ↑ Davies L (6 October 2021). "WHO endorses use of world's first malaria vaccine in Africa". The Guardian. Archived from the original on 7 October 2021. Retrieved 6 October 2021.
- ↑ Drysdale C, Kelleher K. "WHO recommends groundbreaking malaria vaccine for children at risk" (Press release). Geneva: World Health Organization. Archived from the original on 7 October 2021. Retrieved 6 October 2021.
- ↑ Mandavilli A (6 October 2021). "A 'Historical Event': First Malaria Vaccine Approved by W.H.O." New York Times. Archived from the original on 7 October 2021. Retrieved 6 October 2021.
- ↑ Rutgers T, Gordon D, Gathoye AM, Hollingdale M, Hockmeyer W, Rosenberg M, De Wilde M (September 1988). "Hepatitis B Surface Antigen as Carrier Matrix for the Repetitive Epitope of the Circumsporozoite Protein of Plasmodium Falciparum". Nature Biotechnology. 6 (9): 1065–1070. doi:10.1038/nbt0988-1065. S2CID 39880644.
- ↑ RTS,S Clinical Trials Partnership (July 2015). "Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial". Lancet. 386 (9988): 31–45. doi:10.1016/S0140-6736(15)60721-8. PMC 5626001. PMID 25913272.
- ↑ Foquet L, Hermsen CC, van Gemert GJ, Van Braeckel E, Weening KE, Sauerwein R, et al. (January 2014). "Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection". The Journal of Clinical Investigation. 124 (1): 140–4. doi:10.1172/JCI70349. PMC 3871238. PMID 24292709.
- ↑ Swearingen KE, Lindner SE, Shi L, Shears MJ, Harupa A, Hopp CS, et al. (April 2016). "Interrogating the Plasmodium Sporozoite Surface: Identification of Surface-Exposed Proteins and Demonstration of Glycosylation on CSP and TRAP by Mass Spectrometry-Based Proteomics". PLOS Pathogens. 12 (4): e1005606. doi:10.1371/journal.ppat.1005606. PMC 4851412. PMID 27128092.
- ↑ Lopaticki S, Yang AS, John A, Scott NE, Lingford JP, O'Neill MT, et al. (September 2017). "Protein O-fucosylation in Plasmodium falciparum ensures efficient infection of mosquito and vertebrate hosts". Nature Communications. 8 (1): 561. Bibcode:2017NatCo...8..561L. doi:10.1038/s41467-017-00571-y. PMC 5601480. PMID 28916755.
- ↑ Swearingen KE, Lindner SE, Flannery EL, Vaughan AM, Morrison RD, Patrapuvich R, et al. (July 2017). "Proteogenomic analysis of the total and surface-exposed proteomes of Plasmodium vivax salivary gland sporozoites". PLOS Neglected Tropical Diseases. 11 (7): e0005791. doi:10.1371/journal.pntd.0005791. PMC 5552340. PMID 28759593.
- ↑ Goddard-Borger, Ethan D; Boddey, Justin A (1 May 2018). "Implications of Plasmodium glycosylation on vaccine efficacy and design". Future Microbiology. 13 (6): 609–612. doi:10.2217/fmb-2017-0284. ISSN 1746-0913. Archived from the original on 6 February 2023. Retrieved 29 January 2023.
Further reading
- Asante KP, Abdulla S, Agnandji S, Lyimo J, Vekemans J, Soulanoudjingar S, et al. (October 2011). "Safety and efficacy of the RTS,S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial". The Lancet. Infectious Diseases. 11 (10): 741–9. doi:10.1016/S1473-3099(11)70100-1. PMID 21782519.
- Wilby KJ, Lau TT, Gilchrist SE, Ensom MH (March 2012). "Mosquirix (RTS,S): a novel vaccine for the prevention of Plasmodium falciparum malaria". The Annals of Pharmacotherapy. 46 (3): 384–93. doi:10.1345/aph.1AQ634. PMID 22408046. S2CID 32412123. Archived from the original on 27 October 2021. Retrieved 29 January 2023.
External links
- Mosquirix: Product information Archived 20 September 2022 at the Wayback Machine
- Mosquirix: Public Assessment Report Archived 11 October 2022 at the Wayback Machine