Niemann–Pick disease
| Niemann–Pick disease | |
|---|---|
 | |
| a) Normal b) altered cholesterol trafficking in Niemann–Pick disease type C[1] | |
| Pronunciation |
|
| Specialty | Medical genetics |
Niemann–Pick disease is a group of severe inherited metabolic disorders, in which sphingomyelin accumulates in lysosomes in cells (the lysosomes normally degrade material that comes from out of cells).
These disorders involve the dysfunctional metabolism of sphingolipids, which are fats found in cell membranes. They can be considered as a kind of sphingolipidosis, which is included in the larger family of lysosomal storage diseases.[3]
Signs and symptoms

Symptoms are related to the organs in which sphingomyelin accumulates. Enlargement of the liver and spleen (hepatosplenomegaly) may cause reduced appetite, abdominal distension, and pain. Enlargement of the spleen (splenomegaly) may also cause low levels of platelets in the blood (thrombocytopenia).
Accumulation of sphingomyelin in the central nervous system (including the cerebellum) results in unsteady gait (ataxia), slurring of speech (dysarthria), and difficulty swallowing (dysphagia). Basal ganglia dysfunction causes abnormal posturing of the limbs, trunk, and face (dystonia). Upper brainstem disease results in impaired voluntary rapid eye movements (supranuclear gaze palsy). More widespread disease involving the cerebral cortex and subcortical structures causes gradual loss of intellectual abilities, causing dementia and seizures.
Bones also may be affected, with the disease causing enlarged bone marrow cavities, thinned cortical bone, or a distortion of the hip bone called coxa vara. Sleep-related disorders also occur with the condition, such as sleep inversion, sleepiness during the day and wakefulness at night. Gelastic cataplexy, the sudden loss of muscle tone when the affected patient laughs, is also seen.
Causes
Mutations in the SMPD1 gene cause Niemann–Pick disease types A and B. They produce a deficiency in the activity of the lysosomal enzyme acid sphingomyelinase, that breaks down the lipid sphingomyelin.[4]
Mutations in NPC1 or NPC2 cause Niemann–Pick disease, type C (NPC), which affects a protein used to transport lipids.[4]
Type D originally was separated from type C to delineate a group of patients with otherwise identical disorders who shared a common Nova Scotian ancestry. Patients in this group are known to share a specific mutation in the NPC1 gene, so NPC is used for both groups. Before the molecular defects were described, the terms "Niemann–Pick type I" and "Niemann–Pick type II" were proposed to separate the high- and low-sphingomyelin forms of the disease in the early 1980s.
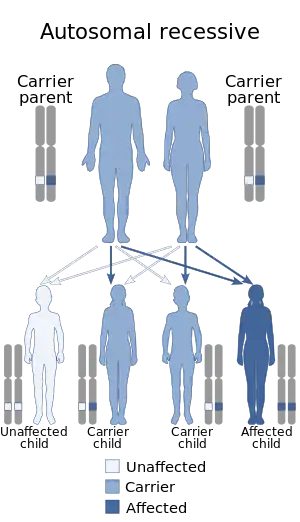
Niemann–Pick disease is inherited in an autosomal recessive pattern,[5][6] which means both copies, or both alleles of the gene, must be defective to cause the disease. "Defective" means they are altered in a way that impairs their function. Most often, the parents of a child with an autosomal recessive disorder are carriers: they have one copy of the altered gene, but are not affected because the other copy produces the enzyme. If both parents are carriers, each pregnancy has a 25% chance of producing an affected child. Genetic counseling and genetic testing are recommended for families who may be carriers of the disease.
Pathophysiology
Niemann–Pick diseases are a subgroup of lipid storage disorders called sphingolipidoses in which harmful quantities of fatty substances, or lipids, accumulate in the spleen, liver, lungs, bone marrow, and brain.
In the classic infantile type-A variant, a missense mutation causes complete deficiency of sphingomyelinase. Sphingomyelin is a component of cell membrane including the organellar membrane, so the enzyme deficiency blocks degradation of lipid, resulting in the accumulation of sphingomyelin within lysosomes in the macrophage-monocyte phagocyte lineage. Affected cells become enlarged, sometimes up to 90 μm in diameter, secondary to the distention of lysosomes with sphingomyelin and cholesterol. Histology shows lipid-laden macrophages in the marrow and "sea-blue histiocytes" on pathology. Numerous small vacuoles of relatively uniform size are created, giving the cytoplasm a foamy appearance.
Diagnosis

For type A and B, levels of sphingomylinase can be measured from a blood sample. To diagnose type C, a skin sample can help determine whether the transporter is affected via the Filipin test which detects build-up of unesterified cholesterol via fluorescent staining.[7] [8]
Classification
The four types of Niemann–Pick disease are divided into categories. Patients with ASM deficiency are classified into types A and B. Type A patients exhibit hepatosplenomegaly in infancy and profound central nervous system involvement, and are unable to survive beyond two years of age. Type B patients also show hepatosplenomegaly and pathologic alterations of their lungs, but usually without the involvement of their central nervous system. Some can develop significant life-threatening complications, including liver failure, hemorrhage, oxygen dependency, pulmonary infections, and splenic rupture. Some develop coronary arterial or valvular heart disease. In a longitudinal natural history study, nearly 20% of the patients died. For those classified into type C, they may have mild hepatosplenomegaly, but their central nervous system is profoundly affected.[9]
- Niemann–Pick disease, SMPD1-associated, which includes types A and B
- Niemann–Pick disease type A: classic infantile
- Niemann–Pick disease type B: visceral
- Niemann–Pick disease, type C: subacute/juvenile, includes types C1 (95% of type C) and C2. Type C is the most common form of the disease[4] Type C2 is a rare form of the disease.[10]
Niemann–Pick disease type D (or Nova Scotia form) is now believed to be the same condition as Niemann–Pick disease type C.[11] Two poorly characterized forms of Niemann–Pick disease have also been described as types E and F.[12]
Treatment
No specific treatment is known for type A, but symptoms are treated.
In adult patients with type B, physicians try to keep cholesterol levels down to normal levels. If statins are used, they monitor liver function. If the spleen is enlarged and platelet levels low, acute episodes of bleeding may require transfusions of blood products. If they have symptoms of interstitial lung disease, they may need oxygen.[13]
Anecdotally, organ transplant has been attempted with limited success. Future prospects include enzyme replacement and gene therapy. Bone marrow transplant has been tried for type B.[9]
In January 2009, Actelion announced the drug miglustat (Zavesca) had been approved in the European Union for the treatment of progressive neurological manifestations in adult patients and pediatric patients with NPC. The drug is available to patients in the United States on an experimental basis. In March 2010, the FDA requested additional preclinical and clinical information regarding Zavesca from Actelion before making a final decision on approving the drug in the United States for NPC.[14]
Olipudase alfa (Xenpozyme) was approved for medical use in Japan in March 2022.[15]
Prognosis
Highly variable, infantile neurovisceral Niemann Pick disease (Type A ASMD) is usually fatal before 3 years of age. In Type B ASMD mortality before adulthood is common. But many patients live well into adulthood and may reach a normal lifespan. Diagnoses have been made in the 7th decade of life. [16] [17] [18] Type C is an entirely different disorder, which also has a highly variable prognosis.
Incidence
The incidence among Ashkenazi Jews is estimated to be about one in 40,000 for type A of Niemann–Pick disease.[4] The incidence of both Niemann–Pick disease types A and B in all other populations is estimated to be one in 250,000.[4] The incidence of Niemann–Pick disease type C is estimated to be one in 150,000.[4]
History
Albert Niemann published the first description of what now is known as Niemann–Pick disease, type A, in 1914. Ludwig Pick described the pathology of the disease in a series of papers in the 1930s.[19][20][21]
In 1961, the classification of Niemann–Pick disease into types A, B, and C was introduced, and also contained a type D,[22][23] called the "Nova Scotian type". Genetic studies showed that type D is caused by the same gene as type C1, and the type D designation is no longer used.[4]
Research
Research has been ongoing to better understand the disease and treatments for it, however at present there is no cure. [24]
The loss of myelin in the central nervous system is considered to be a main pathogenic factor. Research uses animal models carrying the underlying mutation for Niemann–Pick disease, e.g. a mutation in the NPC1 gene as seen in Niemann–Pick type C disease. In this model, the expression of myelin gene regulatory factor (MRF) has been shown to be significantly decreased.[25] MRF is a transcription factor of critical importance in the development and maintenance of myelin sheaths.[26] A perturbation of oligodendrocyte maturation and the myelination process might, therefore, be an underlying mechanism of the neurological deficits.[25]
Curiously, in 2011 fibroblast cells derived from patients with Niemann–Pick type C1 disease were shown to be resistant to Ebola virus because of mutations in the NPC1 protein, which is needed for viral escape from the vesicular compartment.[27]
Other studies have uncovered small molecules which inhibit the receptor and may be a potential therapeutic strategy.[28]
Arimoclomol
In 2014, the European Medicines Agency granted orphan drug designation to arimoclomol for the treatment of Niemann–Pick type C.[29] This was followed in 2015 by the U.S. Food & Drug Administration.[30] Dosing in a placebo-controlled phase II/III clinical trial to investigate treatment for Niemann–Pick type C (for patients with both type C1 and C2) using arimoclomol began in 2016.[31]
2-hydroxypropyl-β-cyclodextrin
Researchers at the University of Arizona first proposed the use of 2-hydroxypropyl-β-cyclodextrins for the treatment of Niemann–Pick Type C1 in 2001.[32] Researchers noted that HPBCDs, with varying levels of 2-hydroxypropyl substitution, had effects in delaying neurological symptoms and in decreasing liver cholesterol storage in a Niemann–Pick mouse model. Later, researchers at the University of Texas Southwestern Medical Center found that when Niemann–Pick type C mice were injected with 2-hydroxypropyl-β-cyclodextrin (HPbCD) when they were seven days old, they showed marked improvement in liver function, much less neurodegeneration, and ultimately, they lived longer lives than the mice that did not receive this treatment. These results suggest HPbCD acutely reverses the storage defect seen in NPC.[33]
In April 2011, the U.S. National Institutes of Health, in collaboration with the Therapeutics for Rare and Neglected Diseases Program,[34] announced they were developing a clinical trial using HPbCD for Niemann–Pick type C1 patients. A clinical trial conducted by Vtesse, LLC began in January 2013, and was completed in March 2017.[35]
On April 26, 2013, the European Medicines Agency granted International Niemann–Pick Disease Alliance, the United Kingdom, orphan designation for HPbCD for the treatment of Niemann–Pick disease, type C.[36]
Gene therapy
Gene therapy is being used clinically to treat genetic diseases, including haemophilia and spinal muscular atrophy. It has been used preclinically, in a mouse model of Niemann–Pick type C, using an adeno-associated virus-derived viral vector, and has been shown to extend lifespan following injection into the lateral ventricles of the neonatal brain.[37] In a separate proof-of-concept study, a similar vector, but with a modified capsid, was injected intravenously into Niemann–Pick type C mice around four weeks of age; this resulted in extended lifespan and improved weight gain.[38] Gene therapy has also been used preclinically in a mouse model of Niemann–Pick type A. Injection into the cisterna magna at seven weeks of age prevented motor and memory impairment and neuronal cell death.[39]
See also
References
- ↑ Cariati, Ida; Masuelli, Laura; Bei, Roberto; Tancredi, Virginia; Frank, Claudio; D’Arcangelo, Giovanna (January 2021). "Neurodegeneration in Niemann–Pick Type C Disease: An Updated Review on Pharmacological and Non-Pharmacological Approaches to Counteract Brain and Cognitive Impairment". International Journal of Molecular Sciences. 22 (12): 6600. doi:10.3390/ijms22126600. ISSN 1422-0067. Archived from the original on 2023-03-31. Retrieved 2023-10-17.
- ↑ "Niemann–Pick". Oxford English Dictionary (3rd ed.). Oxford University Press. September 2005. (Subscription or UK public library membership required.)
- ↑ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. p. 536. ISBN 978-0-7216-2921-6.
- 1 2 3 4 5 6 7 "Neimann-Pick Disease". Genetics Home Reference. NIH. January 2008. Archived from the original on 24 September 2018. Retrieved 2 October 2012.
- ↑ "Niemann-Pick disease". Genetics Home Reference. Archived from the original on 2018-09-24. Retrieved 2023-09-27.
- ↑ "Niemann-Pick - Symptoms and causes". Mayo Clinic. Archived from the original on 2020-08-04. Retrieved 2023-09-27.
- ↑ "Niemann-Pick - Diagnosis and treatment - Mayo Clinic". Mayo Clinic. Archived from the original on 2023-05-17. Retrieved 2023-09-27.
- ↑ Vanier, M. T.; Latour, P. (2015). Laboratory diagnosis of Niemann-Pick disease type C: The filipin staining test. Methods in Cell Biology. Vol. 126. pp. 357–75. doi:10.1016/bs.mcb.2014.10.028. ISBN 9780128000793. PMID 25665455. Archived from the original on 2023-02-12. Retrieved 2023-09-27.
- 1 2 Schuchman, Edward H.; Wasserstein, Melissa P. (2015). "Types a and B Niemann-Pick disease". Best Practice & Research Clinical Endocrinology & Metabolism. 29 (2): 237–247. doi:10.1016/j.beem.2014.10.002. PMC 5347465. PMID 25987176.
- ↑ Sphingomyelinase Deficiency at eMedicine
- ↑ "Niemann Pick Disease Type C". National Organization for Rare Disorders. Archived from the original on 2021-06-16. Retrieved 2023-09-27.
- ↑ "NIEMANN-PICK DISEASE, TYPE B". OMIM. 9 April 2019. 607616. Archived from the original on 26 March 2023. Retrieved 27 September 2023.
- ↑ Sphingomyelinase Deficiency~treatment at eMedicine
- ↑ "Actelion Pharmaceuticals Ltd (CH) - Actelion receives FDA complete response letter for Zavesca (miglustat) for the treatment of Niemann-Pick type C disease" (Press Release). Drugs.com. 9 March 2010. Archived from the original on 3 June 2021. Retrieved 27 September 2023.
- ↑ "Xenpozyme (olipudase alfa) approved in Japan, first and only approved therapy indicated to treat acid sphingomyelinase deficiency" (Press release). Sanofi. 28 March 2022. Archived from the original on 21 May 2022. Retrieved 20 May 2022.
- ↑ Uz E, Cipil H, Turgut FH, Kaya A, Kargili A, Bavbek N, Ali A, Ali K. Niemann-Pick disease type B presenting with hepatosplenomegaly and thrombocytopenia. South Med J. 2008 Nov;101(11):1188. doi: 10.1097/SMJ.0b013e3181836b4c. PMID: 19088546.
- ↑ McGovern MM, Lippa N, Bagiella E, Schuchman EH, Desnick RJ, Wasserstein MP. Morbidity and mortality in type B Niemann-Pick disease. Genet Med 2013;15:618–623.
- ↑ Cassiman D, Packman S, Bembi B, et al. Cause of death in patients with chronic visceral and chronic neurovisceral acid sphingomyelinase deficiency (NiemannPick disease type B and B variant): Literature review and report of new cases. Mol Genet Metab 2016;118:206–213.
- ↑ synd/1029 at Who Named It?
- ↑ Niemann, A. (1914). "Ein unbekanntes Krankheitsbild" [An unknown disease picture]. Jahrbuch für Kinderheilkunde. Neue Folge (in Deutsch). 79: 1–10.
- ↑ Pick, L. (1926). "Der Morbus Gaucher und die ihm ähnlichen Krankheiten (die lipoidzellige Splenohepatomegalie Typus Niemann und die diabetische Lipoidzellenhypoplasie der Milz)" [Gaucher's disease and similar diseases (type Niemann lipoid cell splenohepatomegaly and spleen diabetic lipoid cell hypoplasia)]. Ergebnisse der Inneren Medizin und Kinderheilkunde (in Deutsch). 29: 519–627.
- ↑ Crocker, Allen C. (1961). "The Cerebral Defect in Tay-Sachs Disease and Niemann-Pick Disease". Journal of Neurochemistry. 7: 69–80. doi:10.1111/j.1471-4159.1961.tb13499.x. PMID 13696518. S2CID 22103848.
- ↑ Online Mendelian Inheritance in Man (OMIM): Niemann–Pick Disease, Type C1; NPC1 - 257220
- ↑ Patterson, Marc (1993), Adam, Margaret P.; Mirzaa, Ghayda M.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Niemann-Pick Disease Type C", GeneReviews®, Seattle (WA): University of Washington, Seattle, PMID 20301473, archived from the original on 2023-10-11, retrieved 2023-07-30
- 1 2 Yan, Xin; Lukas, Jan; Witt, Martin; Wree, Andreas; Hübner, Rayk; Frech, Moritz; Köhling, Rüdiger; Rolfs, Arndt; Luo, Jiankai (2011). "Decreased expression of myelin gene regulatory factor in Niemann-Pick type C 1 mouse". Metabolic Brain Disease. 26 (4): 299–306. doi:10.1007/s11011-011-9263-9. PMID 21938520. S2CID 26878522.
- ↑ Koenning, M.; Jackson, S.; Hay, C. M.; Faux, C.; Kilpatrick, T. J.; Willingham, M.; Emery, B. (2012). "Myelin Gene Regulatory Factor is Required for Maintenance of Myelin and Mature Oligodendrocyte Identity in the Adult CNS". Journal of Neuroscience. 32 (36): 12528–12542. doi:10.1523/JNEUROSCI.1069-12.2012. PMC 3752083. PMID 22956843.
- ↑ Carette, Jan E.; Raaben, Matthijs; Wong, Anthony C.; Herbert, Andrew S.; Obernosterer, Gregor; Mulherkar, Nirupama; Kuehne, Ana I.; Kranzusch, Philip J.; Griffin, April M.; Ruthel, Gordon; Cin, Paola Dal; Dye, John M.; Whelan, Sean P.; Chandran, Kartik; Brummelkamp, Thijn R. (2011). "Ebola virus entry requires the cholesterol transporter Niemann–Pick C1". Nature. 477 (7364): 340–343. Bibcode:2011Natur.477..340C. doi:10.1038/nature10348. PMC 3175325. PMID 21866103.
- ↑ Côté, Marceline; Misasi, John; Ren, Tao; Bruchez, Anna; Lee, Kyungae; Filone, Claire Marie; Hensley, Lisa; Li, Qi; Ory, Daniel; Chandran, Kartik; Cunningham, James (2011). "Small molecule inhibitors reveal Niemann–Pick C1 is essential for Ebola virus infection". Nature. 477 (7364): 344–348. Bibcode:2011Natur.477..344C. doi:10.1038/nature10380. PMC 3230319. PMID 21866101.
- ↑ "European Medicines Agency - - EU/3/14/1376". www.ema.europa.eu. 2018-09-17. Archived from the original on 2017-07-28. Retrieved 2023-09-27.
- ↑ "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Archived from the original on 2021-09-01. Retrieved 2023-09-27.
- ↑ "Arimoclomol Prospective Study in Patients Diagnosed With NiemannPick Disease Type C - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. 14 April 2021. Archived from the original on 14 August 2022. Retrieved 27 September 2023.
- ↑ Camargo, Fernando; Erickson, Robert P.; Garver, William S.; Hossain, G.Showkat; Carbone, Peter N.; Heidenreich, Randall A.; Blanchard, James (2001). "Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease". Life Sciences. 70 (2): 131–142. doi:10.1016/S0024-3205(01)01384-4. PMID 11787939.
- ↑ Liu, B.; Turley, S. D.; Burns, D. K.; Miller, A. M.; Repa, J. J.; Dietschy, J. M. (2009). "Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse". Proceedings of the National Academy of Sciences. 106 (7): 2377–2382. doi:10.1073/pnas.0810895106. PMC 2650164. PMID 19171898.
- ↑ "Therapeutics for Rare and Neglected Diseases Program". U.S. National Institutes of Health. November 2017. Archived from the original on 2013-04-09. Retrieved 2023-09-27.
- ↑ "Hydroxypropyl Beta Cyclodextrin for Niemann-Pick Type C1 Disease". ClinicalTrials.gov. NIH. 19 July 2021. Archived from the original on 11 January 2022. Retrieved 10 January 2022.
- ↑ "EU/3/13/1124 | European Medicines Agency". European Medicines Agency. 17 September 2018. Archived from the original on 16 April 2021. Retrieved April 16, 2021.
- ↑ Hughes, Michael P; Smith, Dave A; Morris, Lauren; Fletcher, Claire; Colaco, Alexandria; Huebecker, Mylene; Tordo, Julie; Palomar, Nuria; Massaro, Giulia; Henckaerts, Els; Waddington, Simon N (2018-09-01). "AAV9 intracerebroventricular gene therapy improves lifespan, locomotor function and pathology in a mouse model of Niemann–Pick type C1 disease". Human Molecular Genetics. 27 (17): 3079–3098. doi:10.1093/hmg/ddy212. ISSN 0964-6906. PMC 6097154. PMID 29878115. Archived from the original on 2022-01-24. Retrieved 2023-09-27.
- ↑ Davidson, Cristin D.; Gibson, Alana L.; Gu, Tansy; Baxter, Laura L.; Deverman, Benjamin E.; Beadle, Keith; Incao, Arturo A.; Rodriguez-Gil, Jorge L.; Fujiwara, Hideji; Jiang, Xuntian; Chandler, Randy J. (2021-10-01). "Improved systemic AAV gene therapy with a neurotrophic capsid in Niemann–Pick disease type C1 mice". Life Science Alliance. 4 (10): e202101040. doi:10.26508/lsa.202101040. ISSN 2575-1077. PMC 8380657. PMID 34407999.
- ↑ Samaranch, Lluis; Pérez-Cañamás, Azucena; Soto-Huelin, Beatriz; Sudhakar, Vivek; Jurado-Arjona, Jerónimo; Hadaczek, Piotr; Ávila, Jesús; Bringas, John R.; Casas, Josefina; Chen, Haifeng; He, Xingxuan (2019-08-21). "Adeno-associated viral vector serotype 9–based gene therapy for Niemann-Pick disease type A". Science Translational Medicine. 11 (506): eaat3738. doi:10.1126/scitranslmed.aat3738. ISSN 1946-6234. PMC 7285630. PMID 31434754.
External links
- niemann at NINDS
- Genetics Home Reference on Niemann–Pick Disease Archived 2018-09-24 at the Wayback Machine
- This article incorporates public domain text from The U.S. National Library of Medicine Archived 2019-02-04 at the Wayback Machine
| Classification | |
|---|---|
| External resources |