Sacubitril
 | |
| Clinical data | |
|---|---|
| Other names | AHU-377 |
| License data | |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H29NO5 |
| Molar mass | 411.498 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sacubitril (/səˈkjuːbɪtrɪl/; INN) is an antihypertensive drug used in combination with valsartan. The combination drug sacubitril/valsartan, known during trials as LCZ696 and marketed under the brand name Entresto, is a treatment for heart failure.[1] It was approved under the FDA's priority review process for use in heart failure on July 7, 2015.
Side effects
Sacubitril increases levels of bradykinin, which is responsible for the edema seen sometimes in patients with the medication. This is why the medication is not recommended for patients with a history of pulmonary edema with the usage of ACE inhibitors.
Mechanism of action
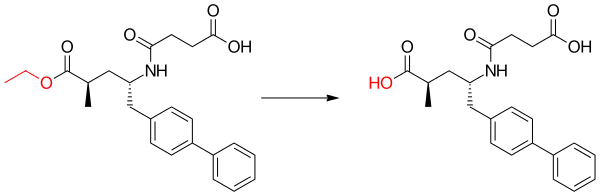
Sacubitril is a prodrug that is activated to sacubitrilat (LBQ657) by de-ethylation via esterases.[2] Sacubitrilat inhibits the enzyme neprilysin,[3] which is responsible for the degradation of atrial and brain natriuretic peptide, two blood pressure–lowering peptides that work mainly by reducing blood volume.[4] In addition, neprilysin degrades a variety of peptides including bradykinin,[5] an inflammatory mediator.
See also
References
- ↑ McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. (September 2014). "Angiotensin-neprilysin inhibition versus enalapril in heart failure". The New England Journal of Medicine. 371 (11): 993–1004. doi:10.1056/NEJMoa1409077. hdl:2336/552372. PMID 25176015.
- ↑ Solomon SD. "HFpEF in the Future: New Diagnostic Techniques and Treatments in the Pipeline". Boston. p. 48. Retrieved 2012-01-26.
- ↑ Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, et al. (April 2010). "Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi)". Journal of Clinical Pharmacology. 50 (4): 401–14. doi:10.1177/0091270009343932. PMID 19934029. S2CID 24853279.
- ↑ Schubert-Zsilavecz M, Wurglics M. "Neue Arzneimittel 2010/2011" (in German).
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Entrez Gene: Membrane metallo-endopeptidase".