Trandolapril
 | |
| Names | |
|---|---|
| Trade names | Mavik, others |
IUPAC name
| |
| Clinical data | |
| Drug class | ACE inhibitor[1] |
| Main uses | High blood pressure, heart failure, diabetic kidney disease[1] |
| Side effects | Cough, diarrhea, dizziness, angioedema[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697010 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | Trandolapril 80% (independent of concentration) Trandolaprilat 65 to 94% (concentration-dependent) |
| Metabolism | Liver |
| Elimination half-life | 6 hours (trandolapril) 10 hours (trandolaprilat) |
| Excretion | Fecal and Kidney |
| Chemical and physical data | |
| Formula | C24H34N2O5 |
| Molar mass | 430.545 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 119 to 123 °C (246 to 253 °F) |
SMILES
| |
InChI
| |
Trandolapril, sold under the brand name Mavik among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It is one of a number of first line agents for high blood pressure.[1] It is taken by mouth.[1]
Common side effects include cough, diarrhea, and dizziness.[1] Other side effects may include high blood potassium, angioedema, kidney problems, low blood pressure, and liver problems.[1] Use during pregnancy may harm the baby.[1] It is an ACE inhibitor.[1]
Trandolapril was patented in 1981, and approved for medical use in 1993.[2] It is available as a generic medication.[3] In the United Kingdom 4 weeks of treatment costs the NHS about £2.60.[3] This amount in the United States costs about 11 USD.[4]
Medical uses
Combination therapy with paricalcitol and trandolapril has been found to reduce fibrosis in obstructive uropathy.[5]
Dosage
The initial dose is generally 0.5 mg per day which is than increased to 1 to 2 mg per day up to 4 mg per day.[3]
Side effects
Side effects reported for trandolapril include nausea, vomiting, diarrhea, headache, dry cough, dizziness or lightheadedness when sitting up or standing, hypotension, or fatigue.
Pregnancy and breastfeeding
Trandolapril is teratogenic (US: pregnancy category D) and can cause birth defects and even death of the developing fetus. The highest risk to the fetus is during the second and third trimesters. When pregnancy is detected, trandolapril should be discontinued as soon as possible. Trandolapril should not be administered to nursing mothers.
Interactions
People also on diuretics may experience an excessive reduction of blood pressure after initiation of therapy with trandolapril. It can reduce potassium loss caused by thiazide diuretics, and increase serum potassium when used alone. Therefore, hyperkalemia is a possible risk. Increased serum lithium levels can occur in patients who are also on lithium.
Mechanism of action
Trandolapril acts by competitive inhibition of angiotensin converting enzyme (ACE), a key enzyme in the renin–angiotensin system which plays an important role in regulating blood pressure.
Pharmacology
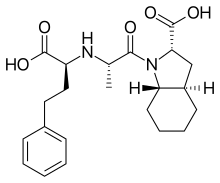
Trandolapril is a prodrug that is de-esterified to trandolaprilat. It is believed to exert its antihypertensive effect through the renin–angiotensin–aldosterone system. Trandolapril has a half-life of about 6 hours, and trandolaprilat has a half life of about 10 h. Trandolaprilat has about eight times the activity of its parent drug. About one-third of trandolapril and its metabolites are excreted in the urine, and about two-thirds of trandolapril and its metabolites are excreted in the feces. Serum protein binding of trandolapril is about 80%.
References
- 1 2 3 4 5 6 7 8 9 10 "Trandolapril Monograph for Professionals". Drugs.com. Archived from the original on 6 March 2021. Retrieved 8 October 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 469. ISBN 9783527607495. Archived from the original on 2021-08-29. Retrieved 2021-01-06.
- 1 2 3 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 185. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Trandolapril Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 8 October 2021.
- ↑ Tan X, He W, Liu Y (December 2009). "Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy". Kidney International. 76 (12): 1248–57. doi:10.1038/ki.2009.346. PMC 5527548. PMID 19759524.
External links
| Identifiers: |
|---|
- Trandolapril Information - rxlist.com Archived 2007-02-12 at the Wayback Machine (Rxlist.com, The Internet Drug Index)