Aliskiren
 | |
| Names | |
|---|---|
| Trade names | Tekturna, Rasilez |
| Other names | Aliskiren hemifumarate |
IUPAC name
| |
| Clinical data | |
| Drug class | Renin inhibitors[1] |
| Main uses | High blood pressure[1] |
| Side effects | Diarrhea, headache, dizziness, cough, rash, high potassium, kidney problems[2] |
| Interactions | NSAIDS, ciclosporine, itraconazole[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607039 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | Low (approximately 2.5%) |
| Metabolism | Liver, CYP3A4-mediated |
| Elimination half-life | 24 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C30H53N3O6 |
| Molar mass | 551.769 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Aliskiren, sold under the brand names Tekturna and Rasilez is a medication used to treat high blood pressure.[1] Other better studied medications are typically recommended; however.[3][2] It is taken by mouth.[4]
Common side effects include diarrhea, headache, dizziness, cough, rash, high potassium, and kidney problems.[2] Other side effects may include low blood pressure and angioedema.[2] Use in pregnancy may harm the baby.[2] It is a renin inhibitors, which prevents the conversion of angiotensinogen to angiotensin I.[1][4]
Aliskiren was approved for medical use in the United States and Europe in 2007.[1][5] It is available as a generic medication.[6] In the United Kingdom 4 weeks costs the NHS about £30 as of 2021.[4] This amount in the United States is about 72 USD.[6]
Medical uses
While used for high blood pressure, other better-studied medications are typically recommended.[3] Prescrire has stated that aliskiren is potentially more harmful than beneficial and thus list it as a drug to avoid (as of 2014).[3]
Dosage
It is generally used at a dose of 150 mg once per day; though occasionally a dose of 300 mg once per day may be used.[4]
Side effects
- Angioedema - The ADE of angioedema found in patients using Aliskiren is due to the inhibition of bradykinin degradation which occurs within the Renin-Angiotensin System (RAAS)
- High blood potassium level (particularly when used with ACE inhibitors in diabetic patients)
- Low blood pressure (particularly in volume-depleted patients)
- Diarrhea and other GI symptoms
- Headache
- Dizziness
- Cough
In December 2011, Novartis halted a trial after discovering increased nonfatal stroke, kidney complications, high blood potassium, and low blood pressure in people with diabetes and kidney problems.[7][8]
As a result, in 2012:
- A new contraindication was added to the product label concerning the use of aliskiren with angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEIs) in patients with diabetes because of the risk of kidney impairment, low blood pressure, and high levels of potassium in the blood.[9]
- A warning to avoid use of aliskiren with ARBs or ACEIs was also added for patients with moderate to severe kidney impairment (i.e., where glomerular filtration rate is less than 60 ml/min).[9]
- Novartis decided to stop marketing Valturna (aliskiren/valsartan).[10]
Contraindications
- Pregnancy: Other drugs such as ACE inhibitors, also acting on the renin–angiotensin system, have been associated with fetal malformations and neonatal death.[11] Angiotensin cannot be used in patients who are pregnant because it will result in disruption of normal fetal kidney development.
- Breastfeeding: During animal studies, the drug has been found present in milk.[11]
- Aliskiren has been shown to increase the likelihood of adverse cardiovascular outcomes in patients with diabetes and kidney or heart disease.[8]
Interactions
Aliskiren is a minor inhibitor of substrate CYP3A4 and, more importantly, P-glycoprotein:
- It reduces furosemide blood concentration.
- Atorvastatin may increase blood concentration, but no dose adjustment is needed.
- Due to possible interaction with ciclosporin, the use of ciclosporin and aliskiren at the same time is contraindicated.
- Caution should be exercised when aliskiren is administered with ketoconazole or other moderate P-glycoprotein inhibitors (itraconazole, clarithromycin, telithromycin, erythromycin, or amiodarone).
- Recommendations have been made to stop prescribing aliskiren-containing medicines to patients with diabetes (type 1 or type 2) or with moderate to severe kidney impairment who are also taking an ACE inhibitor or ARB. Such patients should consider alternative antihypertensive treatment as necessary.[12]
Mechanism of action
Aliskiren is an antagonist to renin.[13] Renin, the first enzyme in the renin–angiotensin–aldosterone system, plays a role in blood pressure control. It cleaves angiotensinogen to angiotensin I, which is in turn converted by angiotensin-converting enzyme (ACE) to angiotensin II. Angiotensin II has both direct and indirect effects on blood pressure. It directly causes arterial smooth muscle to contract, leading to vasoconstriction and increased blood pressure. Angiotensin II also stimulates the production of aldosterone from the adrenal cortex, which causes the tubules of the kidneys to increase reabsorption of sodium, with water following, thereby increasing plasma volume, and thus blood pressure. Aliskiren binds to the S3bp binding site of renin, essential for its activity.[13] Binding to this pocket prevents the conversion of angiotensinogen to angiotensin I. Aliskiren is also available as combination therapy with hydrochlorothiazide.[14]
Chemistry
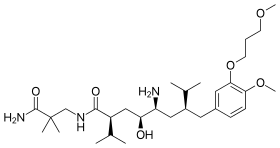
The chemical name for aliskiren is (2 S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2-methylpropyl)-4-hydroxy-2-isopropyl-7-[ 4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methylnonanamide.[15]
History
Many drugs control blood pressure by interfering with angiotensin or aldosterone. However, when these drugs are used chronically, the body increases renin production, which drives blood pressure up again. Therefore, pharmacologists have been looking for a drug to inhibit renin directly. Aliskiren is the first drug to do so.[16][17]
It was co-developed by the Swiss pharmaceutical companies Novartis and Speedel.[18][19]
References
- 1 2 3 4 5 6 "DailyMed - ALISKIREN- aliskiren hemifumarate tablet, film coated". dailymed.nlm.nih.gov. Archived from the original on 23 March 2021. Retrieved 13 January 2022.
- 1 2 3 4 5 "Aliskiren Monograph for Professionals". Drugs.com. Archived from the original on 25 January 2021. Retrieved 14 January 2022.
- 1 2 3 "Towards better patient care: drugs to avoid in 2014". Prescrire International. 23 (150): 161–5. June 2014. PMID 25121155. Archived from the original on 2014-07-14. Retrieved 2021-10-31.
- 1 2 3 4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 194. ISBN 978-0857114105.
- ↑ "Rasilez". Archived from the original on 12 November 2020. Retrieved 13 January 2022.
- 1 2 "Aliskiren Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 14 January 2022.
- ↑ Healthzone.ca: Blood-pressure drug reviewed amid dangerous side effects Archived 2012-05-12 at the Wayback Machine
- 1 2 Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. (December 2012). "Cardiorenal end points in a trial of aliskiren for type 2 diabetes" (PDF). The New England Journal of Medicine. 367 (23): 2204–13. doi:10.1056/NEJMoa1208799. PMID 23121378. Archived (PDF) from the original on 2021-09-14. Retrieved 2021-10-31.
- 1 2 "FDA Drug Safety Communication: New Warning and Contraindication for blood pressure medicines containing aliskiren (Tekturna)". U.S. Food and Drug Administration (FDA). 19 January 2016. Archived from the original on 18 December 2019. Retrieved 12 February 2020.
- ↑ "Aliskiren Information". U.S. Food and Drug Administration. 8 July 2015. Archived from the original on 15 December 2019. Retrieved 12 February 2020.
- 1 2 Drugs.com: Tekturna Archived 2016-03-03 at the Wayback Machine
- ↑ European Medicines Agency recommends new contraindications and warnings for aliskiren-containing medicines.
- 1 2 Rahuel J, Rasetti V, Maibaum J, Rüeger H, Göschke R, Cohen NC, et al. (July 2000). "Structure-based drug design: the discovery of novel nonpeptide orally active inhibitors of human renin". Chemistry & Biology. 7 (7): 493–504. doi:10.1016/S1074-5521(00)00134-4. PMID 10903938.
- ↑ Baldwin CM, Plosker GL (2009). "Aliskiren/hydrochlorothiazide combination: in mild to moderate hypertension". Drugs. 69 (7): 833–41. doi:10.2165/00003495-200969070-00004. PMID 19441870. S2CID 26512682.
- ↑ "Recommended INN List 45" (PDF). WHO Drug Information. 15 (1). 2001. Archived (PDF) from the original on 2021-09-14. Retrieved 2021-10-31.
- ↑ Ingelfinger JR (June 2008). "Aliskiren and dual therapy in type 2 diabetes mellitus". The New England Journal of Medicine. 358 (23): 2503–5. doi:10.1056/NEJMe0803375. PMID 18525047.
- ↑ PharmaXChange: Direct Renin Inhibitors as Antihypertensive Drugs Archived 2010-12-07 at the Wayback Machine
- ↑ Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP (March 2005). "Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients". Circulation. 111 (8): 1012–8. doi:10.1161/01.CIR.0000156466.02908.ED. PMID 15723979.
- ↑ Staessen JA, Li Y, Richart T (October 2006). "Oral renin inhibitors". Lancet. 368 (9545): 1449–56. doi:10.1016/S0140-6736(06)69442-7. PMID 17055947. S2CID 20729350.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Aliskiren at the US National Library of Medicine Medical Subject Headings (MeSH)