Granulomatosis with polyangiitis
| Granulomatosis with polyangiitis | |
|---|---|
| Other names: Wegener's granulomatosis (WG) | |
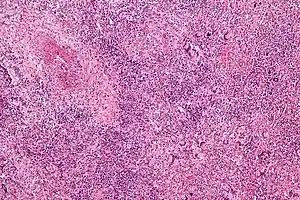 | |
| Micrograph showing features of granulomatosis with polyangiitis – vasculitis and granulomas with multi-nucleated giant cells. H&E stain. | |
| Specialty | Rheumatology |
| Symptoms | Stuffy nose, nosebleeds, inflammation within the eye, fever, coughing up blood, leg swelling, weight loss[1][2] |
| Complications | Hearing loss, blindness, infections, kidney failure[3] |
| Usual onset | 40 to 60 yrs old[2] |
| Causes | Unknown[3] |
| Differential diagnosis | Microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis, polyarteritis nodosa[2] |
| Treatment | Immunosuppressive medications (rituximab, cyclophosphamide, corticosteroids)[4] |
| Prognosis | 90% have significant problems despite treatment[4] |
| Frequency | Rare[3] |
Granulomatosis with polyangiitis (GPA), previously known as Wegener's granulomatosis (WG), is a long-term disorder that involves the formation of granulomas and inflammation of blood vessels (vasculitis).[3][4] It affects small- and medium-size blood vessels, mostly commonly in the upper respiratory tract, lungs, and kidneys.[3] Typical symptoms include a stuffy nose, nosebleeds, and inflammation within the eye.[1] Other symptoms may include fever, coughing up blood, leg swelling, and weight loss.[2] Onset of symptoms may be gradual or rapid.[2] Complications may include hearing loss, blindness, infections, and kidney failure.[3]
The exact cause is unclear, though it is believed to be related to a combination of genetics and environmental exposures.[3][5] It; however, is not inherited from a person's parents.[2] It is a type of autoimmune disorder, specifically a ANCA-associated vasculitis, along with microscopic polyangiitis and eosinophilic granulomatosis with polyangiitis.[3][2] The diagnosis may be suspected based on symptoms and laboratory tests and confirmed by tissue biopsy.[2]
Treatment depends on the severity of the disease.[4][6] Severe disease is typically treated with a combination of immunosuppressive medications such as rituximab or cyclophosphamide and high-dose corticosteroids, to bring about remission of symptoms.[4] To keep the disease under control azathioprine, methotrexate, or rituximab may be used.[4][7] Plasma exchange may be used in cases with kidney injury.[8] Without treatment survival is poor; however, with treatment most live more than 8 years.[3]
The number of new cases a year is estimated at 2–14 per million people in Europe.[1] In the United States about 3 per 100,000 people are affect.[9] Onset is most often between the age of 40 and 60.[2] Males and females are affected with similar frequency.[2] GPA is rare in Japanese and African-American populations.[10] While the condition was first described in 1931 by Heinz Klinger, it was not identified as a separate condition until 1936 by Friedrich Wegener.[3]
Signs and symptoms
.jpg.webp)
Initial signs are highly variable, and diagnosis can be severely delayed due to the nonspecific nature of the symptoms. In general, irritation and inflammation of the nose is the first sign in most people.[12][13] Involvement of the upper respiratory tract, such as the nose and sinuses, is seen in nearly all people with GPA.[14] Typical signs and symptoms of nose or sinus involvement include crusting around the nose, stuffiness, nosebleeds, runny nose, and saddle-nose deformity due to a hole in the septum of the nose.[10][14] Inflammation of the outer layers of the eye (scleritis and episcleritis[15]) and conjunctivitis are the most common signs of GPA in the eye; involvement of the eyes is common and occurs in slightly more than half of people with the disease.[16]
- Kidney: rapidly progressive glomerulonephritis (75%), leading to chronic kidney disease
- Upper airway, eye and ear disease:
- Ears: conductive hearing loss due to auditory tube dysfunction, sensorineural hearing loss (unclear mechanism)
- Oral cavity: strawberry gingivitis, underlying bone destruction with loosening of teeth, non-specific ulcerations throughout the lining of the mouth[17]
- Trachea: subglottal stenosis
- Lungs: pulmonary nodules (referred to as "coin lesions"), infiltrates (often interpreted as pneumonia), cavitary lesions, bleeding in the lungs causing a person to cough up blood, and rarely bronchial stenosis.
- Arthritis: Pain or swelling (60%), often initially diagnosed as rheumatoid arthritis
- Skin: subcutaneous nodules (granulomas) on the elbow, purpura, various others (see cutaneous vasculitis)
- Nervous system: occasionally sensory neuropathy (10%) and rarely mononeuritis multiplex
- Heart, gastrointestinal tract, brain, other organs: rarely affected.
Causes
The cause of GPA is unknown, although microbes, such as bacteria and viruses, as well as genetics have been implicated in its pathogenesis.[13][18]
Pathophysiology
Classic microscopic features of GPA include inflammation of blood vessels associated with poorly formed granulomas, necrosis, and many giant cells.[19] Bacterial colonization with Staphylococcus aureus has been hypothesized as an initiating factor of the autoimmunity seen in people with GPA.[6] Several genes involved in the immune system including PTPN22, CTLA4, and human leukocyte antigen genes may influence the risk of developing GPA.[10]
It is now widely presumed that the anti-neutrophil cytoplasmic antibodies (ANCAs) are responsible for the inflammation in GPA.[12] The typical ANCAs in GPA are those that react with proteinase 3, an enzyme prevalent in neutrophil granulocytes.[10] In vitro studies have found that ANCAs can activate neutrophils, increase their adherence to endothelium, and induce their degranulation that can damage endothelial cells. In theory, this phenomenon could cause extensive damage to the vessel wall, in particular of arterioles.[12]
Diagnosis
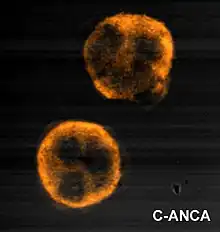
Granulomatosis with polyangiitis is usually suspected only when a person has had unexplained symptoms for a long period of time. Determination of anti-neutrophil cytoplasmic antibodies (ANCAs) can aid in the diagnosis, but positivity is not conclusive and negative ANCAs are not sufficient to reject the diagnosis. More than 90% of people who have GPA test positive for ANCA.[19] Cytoplasmic-staining ANCAs that react with the enzyme proteinase 3 (cANCA) in neutrophils (a type of white blood cell) are associated with GPA.[12] Involvement of the ears, nose, and throat is more common in granulomatosis with polyangiitis than in the similar condition microscopic polyangiitis.[10]
If the person has signs of kidney involvement or cutaneous vasculitis, a biopsy is obtained from the kidneys. On rare occasions, thoracoscopic lung biopsy is required. On histopathological examination, a biopsy will show leukocytoclastic vasculitis with necrotic changes and granulomatous inflammation (clumps of typically arranged white blood cells) on microscopy. These granulomas are the main reason for the name granulomatosis with polyangiitis, although it is not an essential feature. Nevertheless, necrotizing granulomas are a hallmark of this disease. However, many biopsies can be nonspecific and 50% provide too little information for the diagnosis of GPA.[12]
Classification
Granulomatosis with polyangiitis is part of a larger group of vasculitic syndromes called systemic vasculitides or necrotizing vasculopathies, all of which feature an autoimmune attack by an abnormal type of circulating antibody termed ANCAs (antineutrophil cytoplasmic antibodies) against small and medium-size blood vessels. Apart from GPA, this category includes eosinophilic granulomatosis with polyangiitis (EGPA) and microscopic polyangiitis.[7] Although GPA affects small- and medium-size vessels,[20] it is formally classified as one of the small vessel vasculitides in the Chapel Hill system.[21]
Criteria
In 1990, the American College of Rheumatology accepted classification criteria for GPA. These criteria were not intended for diagnosis, but for inclusion in randomized controlled trials. Two or more positive criteria have a sensitivity of 88.2% and a specificity of 92.0% of describing GPA.[14][22]

- Nasal or oral inflammation:
- painful or painless oral ulcers or
- purulent or bloody nasal discharge
- Lungs: abnormal chest X-ray with:
- nodules,
- infiltrates or
- cavities
- Kidneys: urinary sediment with:
- microscopic hematuria or
- red cell casts
- Biopsy: granulomatous inflammation
- within the arterial wall or
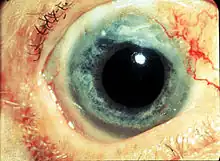 Photo showing the sclerokeratitis associated with GPA
Photo showing the sclerokeratitis associated with GPA - in the perivascular area
According to the Chapel Hill Consensus Conference (CHCC) on the nomenclature of systemic vasculitis (1992), establishing the diagnosis of GPA demands:[23]
- a granulomatous inflammation involving the respiratory tract, and
- a vasculitis of small to medium-size vessels.
Several investigators have compared the ACR and Chapel Hill criteria.[24]
Treatment
GPA treatment depends on its severity and whether it has caused organ damage.[6]
Severe disease
The standard treatment for severe GPA is to induce remission with immunosuppressants such as rituximab or cyclophosphamide in combination with high-dose corticosteroids.[6][25] Plasmapheresis is sometimes recommended for very severe manifestations of GPA, such as diffuse alveolar hemorrhage and rapidly progressive glomerulonephritis (as seen in pulmonary-renal syndrome).[26][27] The use of plasmapheresis in those with GPA and acute kidney failure (renal vasculitis) might reduce progression to end-stage kidney disease at three months.[27]
Oral and intravenous cyclophosphamide are both effective for induction of GPA remission. Oral cyclophosphamide at a dose of 2 mg/kg/day was the standard treatment for many years; this regimen resulted in complete remission in more than 75% of people with GPA but is associated with significant toxicities including infertility, inflammation and bleeding from the bladder, and bladder cancer.[6] In contrast, administering pulsed doses of intravenous cyclophosphamide is equally effective for inducing remission, results in a lower cumulative dose, and decreases the incidence of abnormally low white blood cell counts by one-third.[6] However, pulsed intravenous cyclophosphamide may be associated with a higher risk of GPA relapse when compared to oral cyclophosphamide.[6] Due to a high frequency of abnormally low white blood cell counts seen with cyclophosphamide treatment, Pneumocystis jirovecii pneumonia is a common complication and prophylaxis against this pathogen is recommended.[6]
Rituximab may be substituted for cyclophosphamide to induce remission since it is similarly effective and has a comparable side effect profile.[25][28] The dose of corticosteroids is generally tapered (decreased) very slowly over the course of several months to reduce the risk of another GPA flare. After a person with GPA has successfully undergone induction and gone into remission, the treatment goal then shifts to maintenance of remission and preventing subsequent GPA flares. Less toxic immunosuppressing medications such as rituximab, methotrexate, azathioprine, leflunomide, or mycophenolate mofetil are used.[29] TNF inhibitors, such as etanercept, appear to be ineffective and are not recommended for routine use.[6]
Limited disease
In generalized non-organ-threatening disease, remission can be achieved with a combination of methotrexate and corticosteroids, where the steroid dose is reduced after a remission has been achieved and methotrexate is used as maintenance therapy. Treatment measures for localised GPA of the nose and sinuses includes nasal irrigation, nasal corticosteroids, and antibiotics if infection occurs.[14] If perforation of the nasal septum occurs (or saddle nose deformity), then surgical repair is recommended.[14]
Trimethoprim/sulfamethoxazole has been proposed to help prevent relapse though a 2015 Cochrane review did not confirm fewer relapses with trimethoprim/sulfamethoxazole treatment.[6][27]
Prognosis
Before modern treatments, the 2-year survival was under 10% and average survival five months.[13][30] Death usually resulted from uremia or respiratory failure.[13] The revised Five-factor score is associated with 5-year mortality from GPA and is based on the following criteria: age greater than 65 years, cardiac symptoms, gastrointestinal involvement, chronic kidney disease, and the absence of ears, nose, and throat symptoms.[10]
With corticosteroids and cyclophosphamide, 5-year survival is over 80%.[13] Long-term complications are common (86%), mainly chronic kidney failure, hearing loss, and deafness.[12] The risk of relapse is increased in people with GPA who test positive for anti-PR3 ANCA antibodies and is higher than the relapse risk for microscopic polyangiitis.[10]
Today, medication toxicity is managed more carefully and long-term remissions are possible. Some affected individuals are able to lead relatively normal lives and remain in remission for 20+ years after treatment.[31]
Epidemiology
The incidence is 10–20 cases per million per year.[32][33] It is exceedingly rare in Japan and in African Americans.[33]
History
Scottish otolaryngologist Peter McBride (1854–1946) first described the condition in 1897 in a BMJ article entitled "Photographs of a case of rapid destruction of the nose and face".[34] Heinz Karl Ernst Klinger (born 1907) added information on the anatomical pathology. An early name for the disease was pathergic granulomatosis.[35] The disease is still sometimes confused with lethal midline granuloma and lymphomatoid granulomatosis, both malignant lymphomas.[36]
The full clinical picture was first presented by Friedrich Wegener (1907–1990), a German pathologist, in two reports in 1936 and 1939, leading to the eponymous name Wegener's granulomatosis or Wegener granulomatosis (English: /ˈvɛɡənər/).[9]
In 2006, Alexander Woywodt (Preston, United Kingdom) and Eric Matteson (Mayo Clinic, US) investigated Wegener's past, and discovered that he was, at least at some point of his career, a follower of the Nazi regime. He was a member of the Sturmabteilung paramilitary group and worked in an office where medical experiments were conducted on Jewish people.[37] In addition, their research indicate that Wegener was wanted by Polish authorities and that his files were forwarded to the United Nations War Crimes Commission. Furthermore, Wegener worked in close proximity to the genocide machinery in Łódź. Their research raised serious concerns about Wegener's professional conduct. They suggested that the eponym be abandoned and proposed "ANCA-associated granulomatous vasculitis" as an alternative name.[38] The authors have since campaigned for other medical eponyms to be abandoned as well.[39] In 2011, the American College of Rheumatology (ACR), the American Society of Nephrology (ASN) and the European League Against Rheumatism (EULAR) resolved to change the name to granulomatosis with polyangiitis.[40] The old name remains widely used despite the consensus to adopt the change.[37]
References
- 1 2 3 Yates, M; Watts, R (February 2017). "ANCA-associated vasculitis". Clinical Medicine (Review). 17 (1): 60–64. doi:10.7861/clinmedicine.17-1-60. PMC 6297586. PMID 28148583.
- 1 2 3 4 5 6 7 8 9 10 "Granulomatosis with Polyangiitis". NORD (National Organization for Rare Disorders). Archived from the original on 22 January 2021. Retrieved 11 April 2021.
- 1 2 3 4 5 6 7 8 9 10 Garlapati, P; Qurie, A (January 2021). "Granulomatosis with Polyangiitis". PMID 32491759.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 5 6 "Granulomatosis with Polyangiitis (GPA) - Musculoskeletal and Connective Tissue Disorders". MSD Manual Professional Edition. Archived from the original on 7 May 2021. Retrieved 11 April 2021.
- ↑ "Granulomatosis with polyangiitis | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 18 March 2021. Retrieved 11 April 2021.
- 1 2 3 4 5 6 7 8 9 10 Lally, L; Spiera, R (2015). "Current therapies for ANCA-associated vasculitis". Annual Review of Medicine (Review). 66: 227–40. doi:10.1146/annurev-med-011514-023051. PMID 25341007.
- 1 2 Singer, O; McCune, WJ (May 2017). "Update on maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis". Current Opinion in Rheumatology (Review). 29 (3): 248–53. doi:10.1097/BOR.0000000000000382. PMID 28306595. S2CID 35805200.
- ↑ Walters, GD; Willis, NS; Cooper, TE; Craig, JC (13 January 2020). "Interventions for renal vasculitis in adults". The Cochrane database of systematic reviews. 1: CD003232. doi:10.1002/14651858.CD003232.pub4. PMID 31927782.
- 1 2 Pakalniskis, MG; Berg, AD; Policeni, BA; Gentry, LR; Sato, Y; Moritani, T; Smoker, WR (December 2015). "The Many Faces of Granulomatosis With Polyangiitis: A Review of the Head and Neck Imaging Manifestations". AJR. American Journal of Roentgenology (Review). 205 (6): W619–29. doi:10.2214/AJR.14.13864. PMID 26587951.
- 1 2 3 4 5 6 7 Millet, A; Pederzoli-Ribeil, M; Guillevin, L; Witko-Sarsat, V; Mouthon, L (August 2013). "Antineutrophil cytoplasmic antibody-associated vasculitides: is it time to split up the group?". Annals of the Rheumatic Diseases (Review). 72 (8): 1273–9. doi:10.1136/annrheumdis-2013-203255. PMID 23606701. S2CID 206849855.
- ↑ "Granulomatosis with polyangiitis | DermNet NZ". www.dermnetnz.org. Archived from the original on 18 March 2021. Retrieved 11 April 2021.
- 1 2 3 4 5 6 Seo P, Stone JH (July 2004). "The antineutrophil cytoplasmic antibody-associated vasculitides". The American Journal of Medicine. 117 (1): 39–50. doi:10.1016/j.amjmed.2004.02.030. PMID 15210387.
- 1 2 3 4 5 Berden A, Göçeroglu A, Jayne D, Luqmani R, Rasmussen N, Bruijn JA, Bajema I (January 2012). "Diagnosis and management of ANCA associated vasculitis". BMJ. 344: e26. doi:10.1136/bmj.e26. PMID 22250224. S2CID 206894936.
- 1 2 3 4 5 Kuan, EC; Suh, JD (February 2017). "Systemic and Odontogenic Etiologies in Chronic Rhinosinusitis". Otolaryngologic Clinics of North America (Review). 50 (1): 95–111. doi:10.1016/j.otc.2016.08.008. PMID 27888918.
- ↑ Schonberg, S; Stokkermans, TJ (January 2020). "Episcleritis". PMID 30521217.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Papaliodis, GN (November 2017). "Ophthalmologic manifestations of systemic vasculitis". Current Opinion in Ophthalmology (Review). 28 (6): 613–16. doi:10.1097/ICU.0000000000000422. PMID 28817388. S2CID 36254262.
- ↑ Marzano, AV; Balice, Y; Tavecchio, S; Desimine, C; Colombo, A; Berti, E (April 2015). "Granulomatous vasculitis". Giornale Italiano di Dermatologia e Venereologia (Review). 150 (2): 193–202. PMID 25791629.
- ↑ Tracy, CL; Papadopoulos, PJ; Bye, MR; Connolly, H; Goldberg, E; O'Brian, RJ; Sharma, GD; Talavera, F; Toder, DS; Valentini, RP; Windle, ML; Wolf, RE (10 February 2014). Diamond, HS (ed.). "Granulomatosis with Polyangiitis". Medscape Reference. WebMD. Archived from the original on 15 July 2021. Retrieved 16 March 2014.
- 1 2 Chen, JH; Deshpande, V (June 2017). "IgG4-related Disease and the Liver". Gastroenterology Clinics of North America (Review). 46 (2): 195–216. doi:10.1016/j.gtc.2017.01.001. PMID 28506361.
- ↑ Gota, CE (May 2013). "Granulomatosis with Polyangiitis (GPA): Vasculitis". Merck Manual Professional. Merck Sharp & Dohme Corp. Archived from the original on 16 March 2014. Retrieved 16 March 2014.
- ↑ Lopalco, G; Rigante, D; Venerito, V; Emmi, G; Anelli, MG; Lapadula, G; Iannone, F; Cantarini, L (June 2016). "Management of Small Vessel Vasculitides". Current Rheumatology Reports (Review). 18 (6): 36. doi:10.1007/s11926-016-0580-1. PMID 27118389. S2CID 21560794.
- ↑ Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, Calabrese LH, Fries JF, Lie JT, Lightfoot RW (August 1990). "The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis". Arthritis and Rheumatism. 33 (8): 1101–7. doi:10.1002/art.1780330807. PMID 2202308.
- ↑ Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG (February 1994). "Nomenclature of systemic vasculitides. Proposal of an international consensus conference". Arthritis and Rheumatism. 37 (2): 187–92. doi:10.1002/art.1780370206. PMID 8129773.
- ↑ Bruce IN, Bell AL (April 1997). "A comparison of two nomenclature systems for primary systemic vasculitis". British Journal of Rheumatology. 36 (4): 453–8. doi:10.1093/rheumatology/36.4.453. PMID 9159539.
- 1 2 Schönermarck, U; Gross, WL; de Groot, K (January 2014). "Treatment of ANCA-associated vasculitis". Nature Reviews. Nephrology (Review). 10 (1): 25–36. doi:10.1038/nrneph.2013.225. PMID 24189648. S2CID 8483900.
- ↑ Keller, SF; Miloslavsky, EM (February 2016). "Corticosteroids in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis". Rheumatic Disease Clinics of North America. 42 (1): 91–101. doi:10.1016/j.rdc.2015.08.010. PMID 26611553.
- 1 2 3 Walters, G; Willis, NS; Craig, JC (September 2015). "Interventions for renal vasculitis in adults". Cochrane Database of Systematic Reviews. 9 (9): CD003232. doi:10.1002/14651858.CD003232.pub3. PMID 26400765.
- ↑ Stone, John H.; Merkel, Peter A.; Spiera, Robert; Seo, Philip; Langford, Carol A.; Hoffman, Gary S.; Kallenberg, Cees G.M.; St. Clair, E. William; Turkiewicz, Anthony; Tchao, Nadia K.; Webber, Lisa (2010-07-15). "Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis". New England Journal of Medicine. 363 (3): 221–232. doi:10.1056/NEJMoa0909905. ISSN 0028-4793. PMC 3137658. PMID 20647199.
- ↑ Tracy, CL; Papadopoulos, PJ; Bye, MR; Connolly, H; Goldberg, E; O'Brian, RJ; Sharma, GD; Talavera, F; Toder, DS; Valentini, RP; Windle, ML; Wolf, RE (10 February 2014). Diamond, HS (ed.). "Granulomatosis with Polyangiitis Treatment & Management". Medscape Reference. WebMD. Archived from the original on 16 March 2014. Retrieved 16 March 2014.
- ↑ Smith RM, Jones RB, Jayne DR (April 2012). "Progress in treatment of ANCA-associated vasculitis". Arthritis Research & Therapy. 14 (2): 210. doi:10.1186/ar3797. PMC 3446448. PMID 22569190.
- ↑ "Vasculitis Foundation " Granulomatosis with Polyangiitis (GPA/Wegener's)". www.vasculitisfoundation.org. Archived from the original on 2014-09-14. Retrieved 2016-03-16.
- ↑ Bosch X, Guilabert A, Espinosa G, Mirapeix E (August 2007). "Treatment of antineutrophil cytoplasmic antibody associated vasculitis: a systematic review". JAMA. 298 (6): 655–69. doi:10.1001/jama.298.6.655. PMID 17684188.
- 1 2 Cartin-Ceba R, Peikert T, Specks U (December 2012). "Pathogenesis of ANCA-associated vasculitis". Current Rheumatology Reports. 14 (6): 481–93. doi:10.1007/s11926-012-0286-y. PMID 22927039. S2CID 12082375.
- ↑ Friedmann I (January 1982). "McBride and the midfacial granuloma syndrome. (The second 'McBride Lecture', Edinburgh, 1980)". The Journal of Laryngology and Otology. 96 (1): 1–23. doi:10.1017/s0022215100092197. PMID 7057076.
- ↑ Fienberg R (December 1955). "Pathergic granulomatosis". The American Journal of Medicine. 19 (6): 829–31. doi:10.1016/0002-9343(55)90150-9. PMID 13275478.
- ↑ Mendenhall WM, Olivier KR, Lynch JW, Mendenhall NP (April 2006). "Lethal midline granuloma-nasal natural killer/T-cell lymphoma". American Journal of Clinical Oncology. 29 (2): 202–6. doi:10.1097/01.coc.0000198738.61238.eb. PMID 16601443. S2CID 25978459.
- 1 2 Lubitz MG (February 2018). "Granulomatosis With Polyangiitis-A Moral Impetus for Change". JAMA Otolaryngology–Head & Neck Surgery. 144 (2): 101. doi:10.1001/jamaoto.2017.2140. PMID 29121164.
- ↑ Woywodt A, Matteson EL (October 2006). "Wegener's granulomatosis—probing the untold past of the man behind the eponym". Rheumatology. 45 (10): 1303–6. doi:10.1093/rheumatology/kel258. PMID 16887845.
- ↑ Woywodt A, Matteson E (September 2007). "Should eponyms be abandoned? Yes". BMJ. 335 (7617): 424. doi:10.1136/bmj.39308.342639.AD. PMC 1962844. PMID 17762033.
- ↑ Falk RJ, Gross WL, Guillevin L, Hoffman G, Jayne DR, Jennette JC, Kallenberg CG, Luqmani R, Mahr AD, Matteson EL, Merkel PA, Specks U, Watts R (April 2011). "Granulomatosis with polyangiitis (Wegener's): an alternative name for Wegener's granulomatosis". Annals of the Rheumatic Diseases. 70 (4): 704. doi:10.1136/ard.2011.150714. PMID 21372195. S2CID 12409848.
External links
| Classification | |
|---|---|
| External resources |