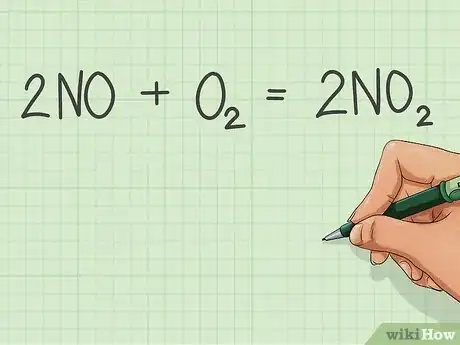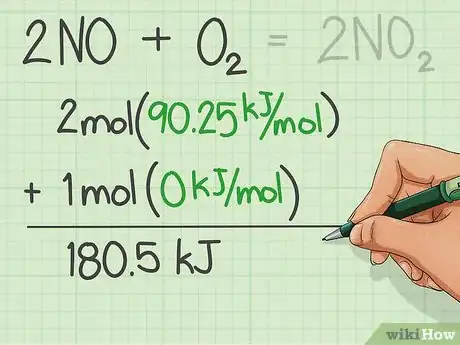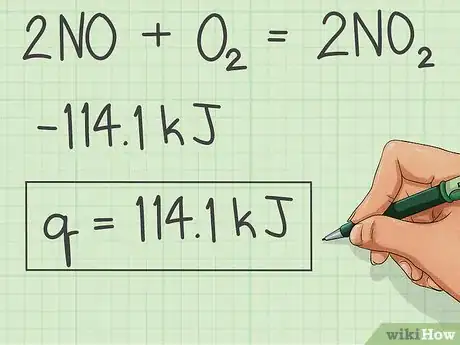wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, volunteer authors worked to edit and improve it over time.
This article has been viewed 137,761 times.
Learn more...
Calculating the heat of combustion is a useful tool in analyzing fuels in terms of energy. The Heat of Combustion of a substance is defined as the amount of energy in the form of heat is liberated when an amount of the substance undergoes combustion.[1]
Steps
Calculating Heat of Combustion Experimentally
-
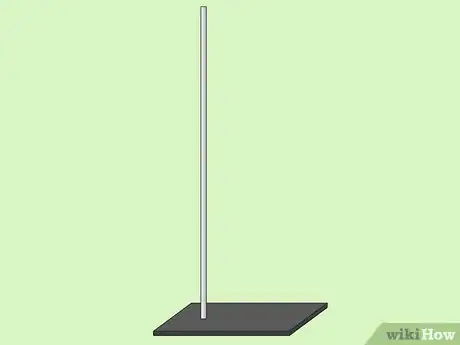
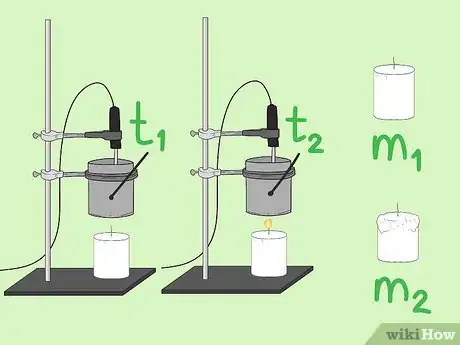
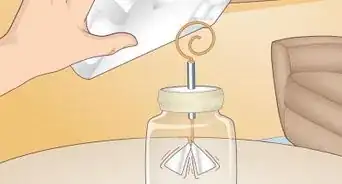
1Position the standing rod vertically. To begin setting up your experiment you will first place the rod on your work table.
-
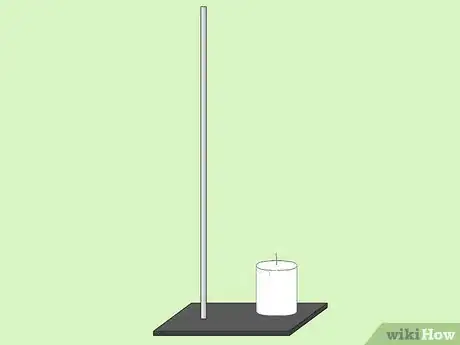
2Measure 100ml of water into the tin can.Advertisement
-
3Put the substance at the base of the standing rod.
-
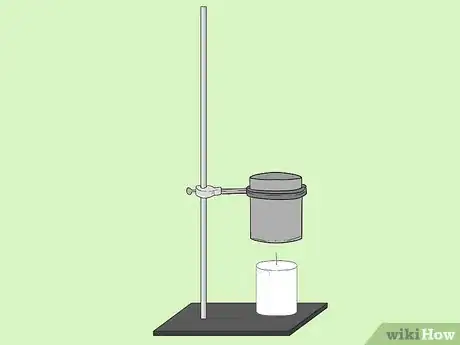
4At 5cm above the substance affix the tin can with a clamp to the rod.
-
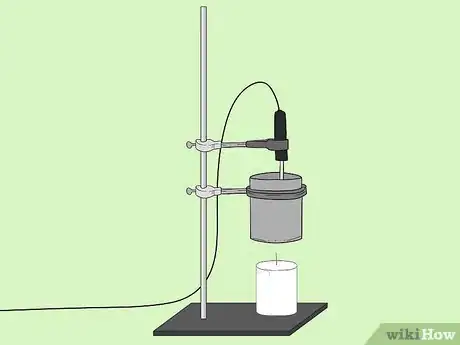
5Affix the thermometer so that it is in the tin can but not touching the bottom base.[2]
-
6Experimentation
- Measure the temperature of the water and note it in degrees celsius.
- Measure the mass of the candle and note it in g.
- Light the substance.
- When the temperature of the water reaches 40 degrees Centigrade, blow out the substance.
- Measure the mass of the candle after burning and note it.
-
7Calculation
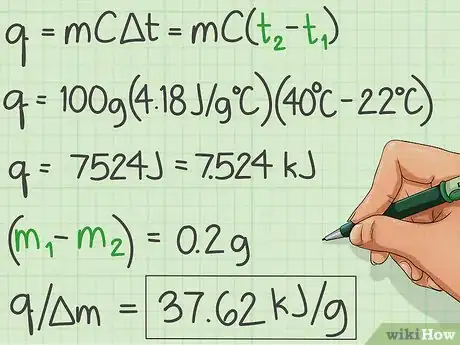
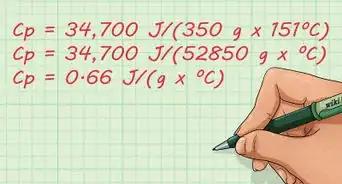
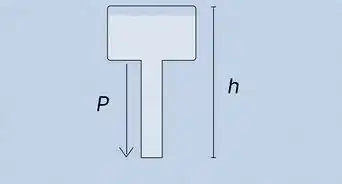
- Use the formula q = Cp * m * (delta) t to calculate the heat liberated which heats the water.[3]
- The specific heat Cp of water is 4.18 J/g C
- Mass of the water is 100g
- Delta t is the difference between the initial starting temperature and 40 degrees centigrade.
- Subtract the initial temperature of the water from 40 C.
- Substitute it into the formula and you will get the answer q in J. Convert into kJ by dividing q by 1000.
- Find the amount of substance burned by subtracting the final mass from the initial mass of the substance in g.
- Divide q in kJ by the mass of the substance burned.
- The answer is the experimental heat of combustion in kJ/g.
Calculating the Heat of Combustion Using Hess' Law
-
1Write the balanced equation of combustion of the substance.[4]
-
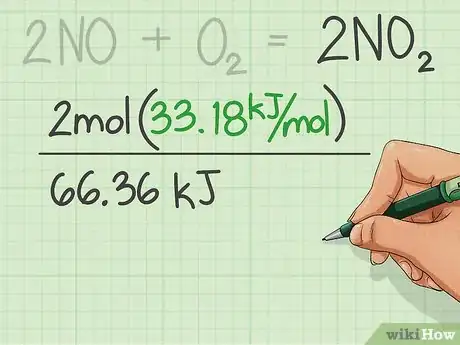
2Find the enthalpies of formation for the reactants and add them.
- Enthalpies of formation are usually found in a table from CRC Handbook of Chemistry and Physics.
-
3Find the enthalpies of formation of the products and add them. Use the table
-
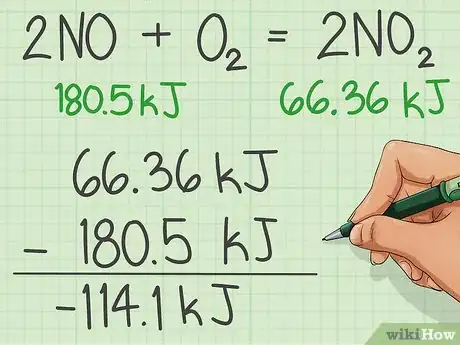
4Subtract the enthalpies of the reactants from the product.
-
5Switch the sign and that is the Heat of Combustion.
Warnings
- The Experimental heat of combustion is inaccurate because it does not factor in heat loss to surrounding environment. It is only a rough estimate.⧼thumbs_response⧽
Things You'll Need
- Gathering Materials
- A tin can
- A standing rod
- A thermometer
- Graduated Cylinder
- Ice cold water
- Two clamps
- The substance - which can be preferable burnt with a wick.
References
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/17%3A_Thermochemistry/17.14%3A_Heat_of_Combustion
- ↑ https://courses.lumenlearning.com/boundless-chemistry/chapter/calorimetry/
- ↑ https://sciencing.com/calculate-heat-absorption-6641786.html
- ↑ https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_Chemistry_Supplement_(Eames)/Thermochemistry/Hess'_Law_and_Enthalpy_of_Formation
- ↑ https://ch301.cm.utexas.edu/section2.php?target=thermo/thermochemistry/hess-law.html
About This Article
The heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. To calculate the heat of combustion, use Hess’s law, which states that the enthalpies of the products and the reactants are the same. Start by writing the balanced equation of combustion of the substance. Then, add the enthalpies of formation for the reactions. You can find these in a table from the CRC Handbook of Chemistry and Physics. After that, add the enthalpies of formation of the products. Next, subtract the enthalpies of the reactants from the product. Finally, change the sign to kilojoules. For more tips, including how to calculate the heat of combustion with an experiment, read on.