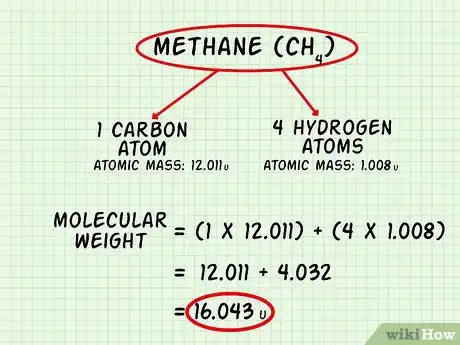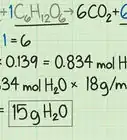This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 7 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 147,051 times.
The molecular mass, often called the molecular weight (MW), is the weight of all atoms in a given molecular formula. Molecular weight is measured in Atomic Mass Units, usually expressed as u or amu.[1] In order to calculate the molecular weight of a formula, you'll need to add up the atomic masses of each element present.
Steps
Calculating Molecular Weight
-
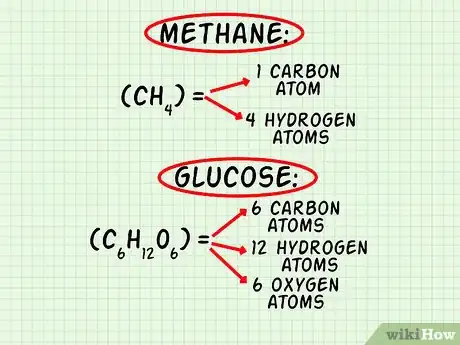
1Count how many atoms of each element exist in the molecule. First, list each element present in the molecule. You may use the chemical symbol or write out the name of the element. Then, count the atoms according to the molecular formula and write them next to the element’s name or symbol.[2]
- For example, carbon dioxide or CO2, list that there is 1 carbon (C) and 2 oxygens (O) present in the molecule.
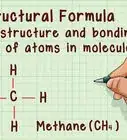
- For methane, CH4, list carbon (C) and hydrogen (H). Methane comprises 1 atom of carbon and 4 atoms of hydrogen.
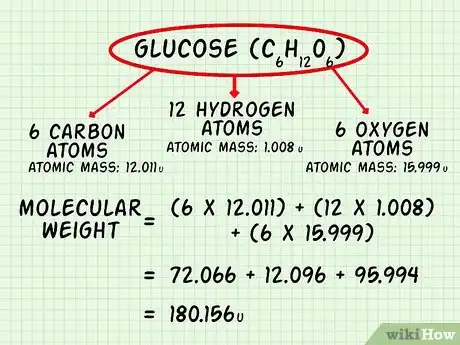
- For glucose, C6H12O6, list carbon (C), hydrogen (H), and oxygen (O). Glucose comprises 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen.
-
2Find the relative atomic mass of each element in the molecule. Use a copy of the Periodic Table of Elements. The Periodic Table lists the atomic mass of each element below the chemical symbol. For example, oxygen has a relative atomic mass of 15.9994 amu.[3]
- For carbon dioxide (CO2), the relative atomic mass is 12.011 amu for carbon and 15.999 for oxygen.
- The atomic mass of an element is roughly equal to the summed mass of the protons and neutrons that it contains. Note that the relative atomic mass you find in the periodic table is scaled: it accounts for all of the isotopes of the element in the proportions that they naturally occur.[4]
Advertisement -
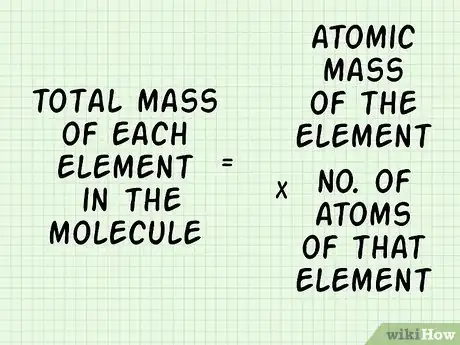
3Calculate the total mass for each element in the molecule. Multiply the atomic mass of each element by the number of atoms of that element: (Atomic Mass of Element) x (# of atoms of that element). Do this for each element in the molecule.[5]
- In our carbon dioxide example, the mass of the single carbon atom is 12.011 amu. Since there are 2 oxygen atoms, you would write .
-
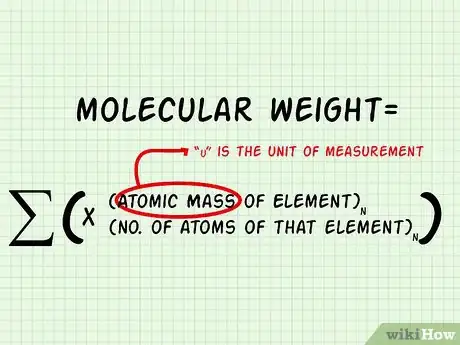
4Add up the mass of all the atoms to find the molecular weight. Molecular weight = ∑((Atomic Mass of Element)n x (# of atoms of that element)n)[6]
Practicing with Examples
-
1
-
2Calculate the molecular weight of glucose (C6H12O6). The atomic mass of carbon is 12.011 u. The atomic mass of hydrogen is 1.008 u. The atomic mass of oxygen is 15.999[10] There are 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. Add up the mass of all of the atoms in glucose to calculate the molecular weight.
Community Q&A
-
QuestionWhat is the molecular mass of phosphorus?
 Community AnswerThe molecular mass of Phosphorus is 30.974 to 3 decimal places, commonly written as 31 on the periodic table.
Community AnswerThe molecular mass of Phosphorus is 30.974 to 3 decimal places, commonly written as 31 on the periodic table. -
QuestionWhy are the hydrogen bonds weak?
 Community AnswerHydrogen has a low electronegativity, and thus is easily taken out of bonds.
Community AnswerHydrogen has a low electronegativity, and thus is easily taken out of bonds. -
QuestionWhat is the molecular weight of CO2?
 Williamsm917Community Answer44.01 g/mol. C has a molecular mass of 12.01. O has a molecular mass of 16. Therefore, the formula would be 12.01 + 2(16), which gives you the molecular mass 44.01 g/mol.
Williamsm917Community Answer44.01 g/mol. C has a molecular mass of 12.01. O has a molecular mass of 16. Therefore, the formula would be 12.01 + 2(16), which gives you the molecular mass 44.01 g/mol.
References
- ↑ http://www.chemteam.info/Mole/MolecWt.html
- ↑ https://www.cmu.edu/gelfand/lgc-educational-media/polymers/what-is-polymer/molecular-weight-calculation.html
- ↑ http://www.ptable.com/
- ↑ https://www.khanacademy.org/science/biology/chemistry--of-life/elements-and-atoms/a/atomic-number-atomic-mass-and-isotopes-article
- ↑ https://chem.libretexts.org/Courses/University_of_British_Columbia/CHEM_100%3A_Foundations_of_Chemistry/06%3A_Chemical_Composition/6.9%3A_Calculating_Molecular_Formulas_for_Compounds
- ↑ http://chemcollective.org/activities/tutorials/stoich/calculating_molecular_weight
- ↑ http://www.chemteam.info/Mole/MolecWt.html
- ↑ http://www.ptable.com/
- ↑ https://www.khanacademy.org/science/chemistry/atomic-structure-and-properties/introduction-to-compounds/v/molecular-mass-and-molecular-weight
About This Article
To calculate molecular weight, start by listing all elements present in the molecule. Then, count the atoms using the molecular formula. For example, use the formula CH4 for methane, which comprises 1 atom of carbon and 4 atoms of hydrogen. In a copy of the Periodic Table of Elements, find the relative atomic mass of each element in the molecule. Then, multiply the atomic mass of an element by the number of atoms of the element in the molecule. To complete the calculation, add up the mass of all of the atoms to get the molecular weight. If you want to learn the units to use when you label your calculations, keep reading the article!