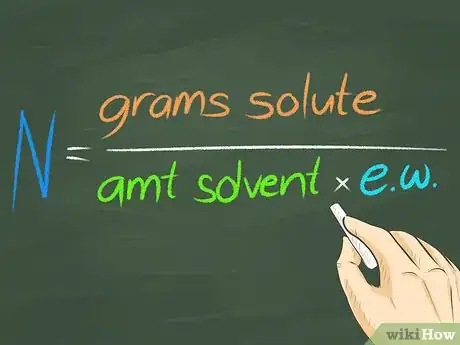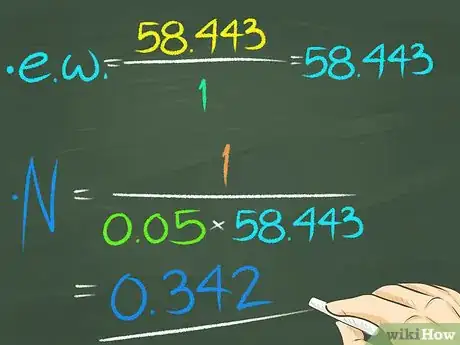This article was co-authored by wikiHow staff writer, Hunter Rising. Hunter Rising is a wikiHow Staff Writer based in Los Angeles. He has more than three years of experience writing for and working with wikiHow. Hunter holds a BFA in Entertainment Design from the University of Wisconsin - Stout and a Minor in English Writing.
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, 80% of readers who voted found the article helpful, earning it our reader-approved status.
This article has been viewed 438,189 times.
Learn more...
Many chemical substances are available in a dissolved liquid form, rather than a solid form. Liquid chemicals are easier to dispense and measure than solid ones, especially since the solid form is usually a powder. However, the stoichiometry of chemical reactions is complicated by the liquid delivery method. Stoichiometry performs calculations using the amount of the desired substance being placed into the equations. The liquid used to dissolve the substance will not participate in the reaction, and stoichiometry will not take that liquid in to account in the reaction. The amount of the reacting substance being delivered can be determined by finding the normality of the solution.[1] Use these tips to learn how to calculate normality.
Steps
-
1Gather information about the equivalent weight of the reacting substance. Consult chemical reference books to find out the valence and the molecular weight of the substance. Molecular weight is the ratio of the mass of 1 molecule of the substance to the mass (one carbon12 molecule divided by 12.) Valence is determined by the maximum number of subatomic valence or atom to atom bonds that the substance may form with other substances.[2] This information will be required to determine normality.
-
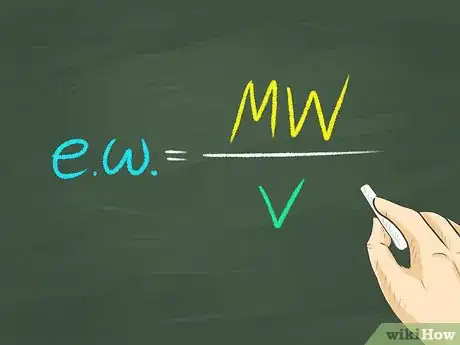
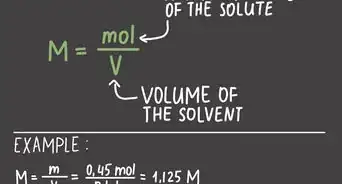
2Figure the equivalent weight of the substance. The equivalent weight of the substance is equal to the molecular weight divided by the valence.[3]Advertisement
-
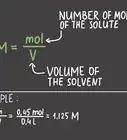
3Calculate normality. Normality is the concentration of the substance of interest in a dissolving liquid. Therefore, normality is a property of the mixture, and will vary with the use of more or less dissolving liquid to place the substance of interest into a solution.[4] Normality is the number of grams of the substance of interest divided by (the equivalent weight of the substance times the amount of dissolving liquid).[5]
-
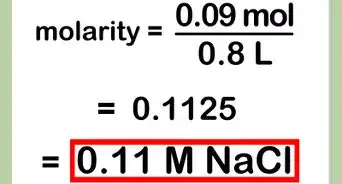
4Try an example.[6] Dissolve sodium chloride (NaCl) in water. Sodium chloride has a valence of 1 and a molecular weight of 58.443. Therefore, the equivalent weight is 58.443/1 or 58.443. 1 gram of NaCl is dissolved into 0.05 L of water, so the normality of the solution is 1/(58.443 x 0.05) or 0.342.
Community Q&A
-
QuestionHow do I calculate my normality?
 Community AnswerYou can use the formula N= weight of substance in grams*1000/ equivalent weight of substance*volume required in mL.
Community AnswerYou can use the formula N= weight of substance in grams*1000/ equivalent weight of substance*volume required in mL. -
QuestionHow do I prepare a 2N HCl solution?
 Community AnswerWeigh 7.29g of HCl in a 100ml volumetric flask. Add deionized water to 100ml and shake for a 2N HCl solution.
Community AnswerWeigh 7.29g of HCl in a 100ml volumetric flask. Add deionized water to 100ml and shake for a 2N HCl solution. -
Question3.65 g of HCl is present in 100mL solution. What is its normality?
 Community AnswerIt's 1N, 1 molecular weight in grams per 1 liter (1000 mL) solution. Molecular weight of HCl is 1 (H) + 35.5 (Cl) = 36.5.
Community AnswerIt's 1N, 1 molecular weight in grams per 1 liter (1000 mL) solution. Molecular weight of HCl is 1 (H) + 35.5 (Cl) = 36.5.
References
- ↑ https://sciencenotes.org/how-to-calculate-normality-of-a-solution/
- ↑ https://sciencing.com/calculate-valence-8566718.html
- ↑ https://sciencing.com/calculate-equivalent-weight-6463740.html
- ↑ https://www.softschools.com/formulas/chemistry/normality_formula/108/
- ↑ https://psiberg.com/how-to-find-normality/
- ↑ https://sciencing.com/calculate-equivalent-units-6224681.html