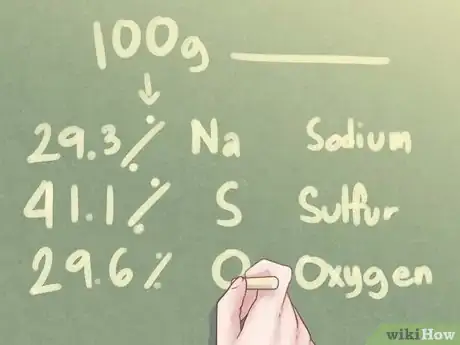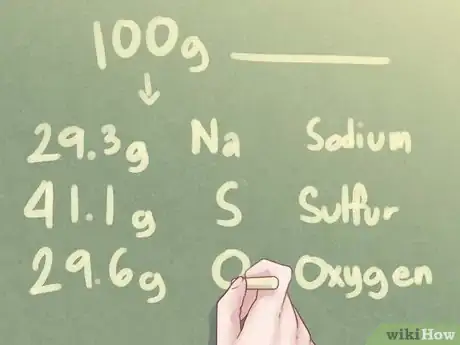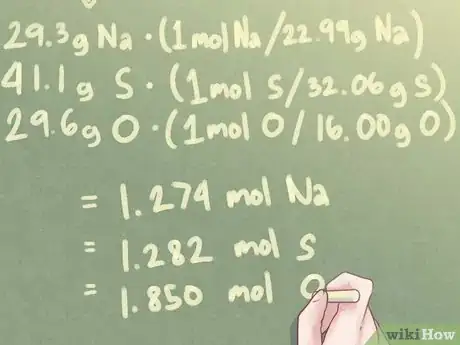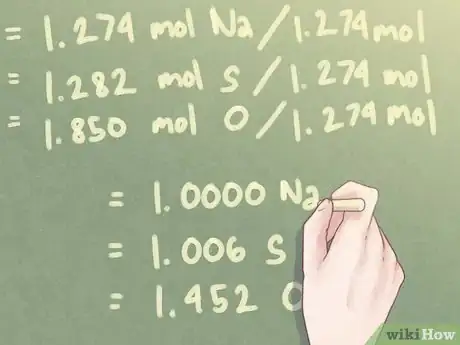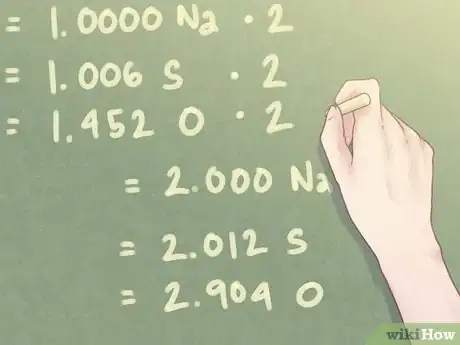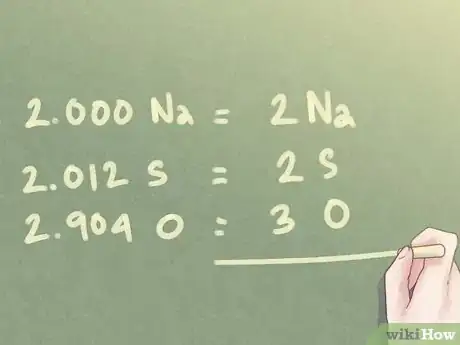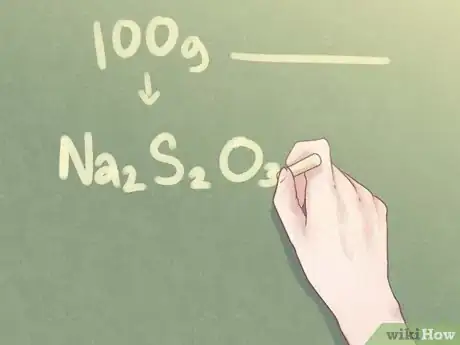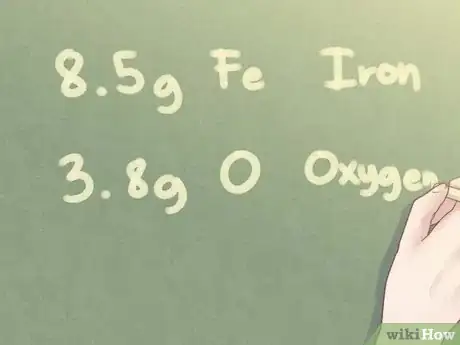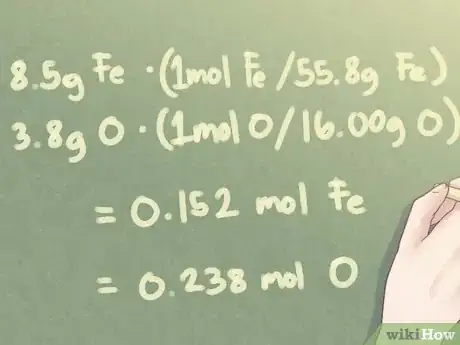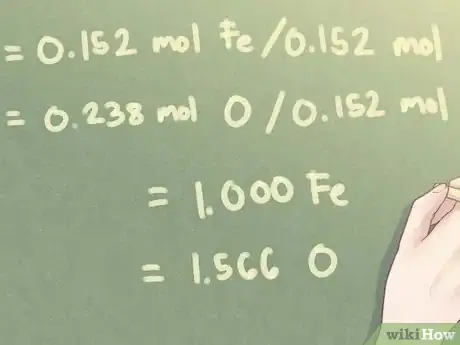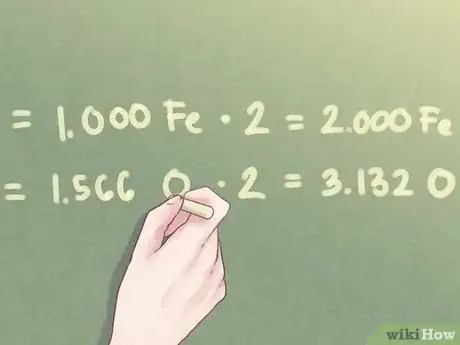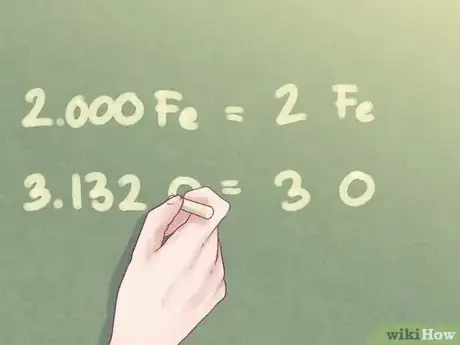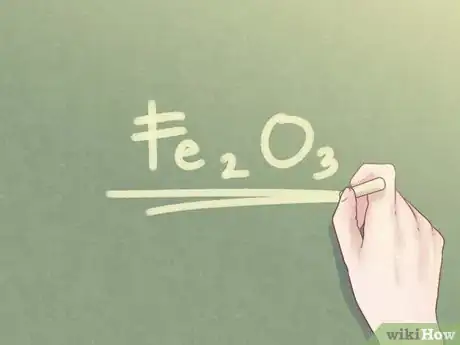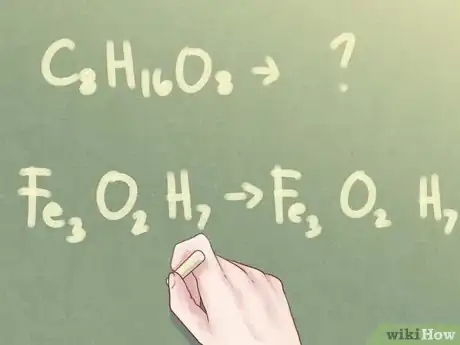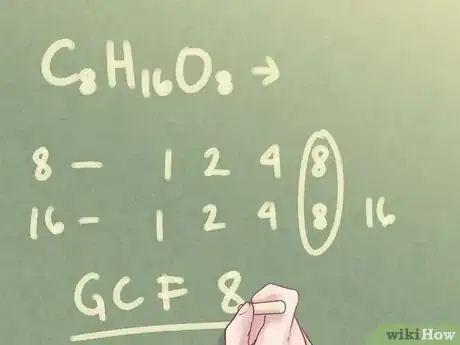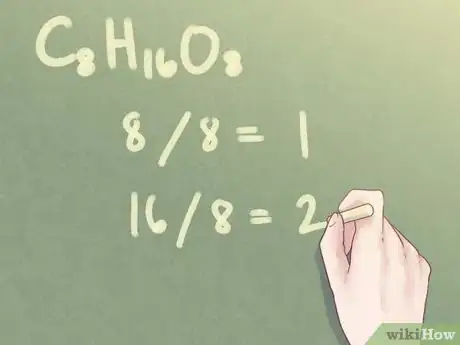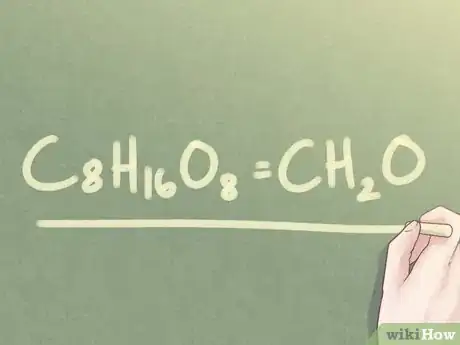wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, volunteer authors worked to edit and improve it over time.
There are 7 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 69,031 times.
Learn more...
A compound's empirical formula is the simplest written expression of its elemental composition. You should be able to determine the empirical formula for any compound as long as you know the mass of each element present, the percentage of mass for each present element, or the molecular formula of the compound.[1]
Steps
Method One: Using Weight Percentages
-
1Look at the data. If you are given the elemental composition of an unknown compound in percentages rather than grams, you should assume that there are exactly 100.0 grams of the substance involved.[2]
- These are the instructions you should follow if the above is true. If you are given the elemental composition of an unknown substance in grams, see the section on "Using Weight in Grams."
- Example: Determine the empirical formula of a compound made from 29.3% Na (sodium), 41.1% S (sulfur), and 29.6% O (oxygen).
-
2Determine the number of grams for each element. Based on the assumption that there are 100 grams of the unknown substance, you can determine that the number of grams present for each element equals the percentage value of each element mentioned in the problem.[3]
- Example: For 100 g of unknown substance, there are 29.3 g Na, 41.1 g S, and 29.6 g O.
Advertisement -
3Convert the mass of each element to moles. The mass of each element in your composition, presently expressed in grams, will need to be converted into moles. To do so, each mass must be multiplied by the mole ratio per their respective atomic weights.[4]
- In simpler terms, you will need to divide each mass by the atomic weight of that element.
- Also note that the atomic weights used in this calculation should include at least four significant figures.
-
Example: For a compound with 29.3 g Na, 41.1 g S, and 29.6 g O:
- 29.3 g Na * (1 mol S / 22.99 g Na) = 1.274 mol Na
- 41.1 g S * (1 mol S / 32.06 g S) = 1.282 mol S
- 29.6 g O * (1 mol O / 16.00 g O) = 1.850 mol O
-
4Divide each mole value by the smallest number of moles present. You will need a stochiometric comparison between the elements in your compound, which essentially means that you need to calculate how much of an element you have in relation to the other elements present in your compound. To do this, divide each number of moles by the smallest number of moles present.[5]
-
Example: The smallest number of moles present in the compound is 1.274 moles (the number of moles for Na, sodium).
- 1.274 mol Na / 1.274 mol = 1.000 Na
- 1.282 mol S / 1.274 mol = 1.006 S
- 1.850 mol O / 1.274 mol = 1.452 O
-
Example: The smallest number of moles present in the compound is 1.274 moles (the number of moles for Na, sodium).
-
5Multiply the ratio values to find near whole numbers. The amount of moles present for each element may not equal whole numbers. For small values that are within a tenth value away from a whole number, this does not present a problem. Once you have an excess value exceeding this amount, however, you should multiply the ratio values as needed to bring that value up to a whole number.[6]
- If one element has a value near 0.5, multiply each element by 2. Similarly, if one element has a value near 0.25, multiply each element by 4.
-
Example: Since the amount of oxygen (O) present is close to 1.5, you will need to multiply each value by “2” to bring the ratio of oxygen closer to a whole number.
- 1.000 Na * 2 = 2.000 Na
- 1.006 S * 2 = 2.012 S
- 1.452 O * 2 = 2.904 O
-
6Round the values to nearest whole numbers. Even after the last step, the amount of moles present for each element may not be in exact whole numbers. Since no decimals are used in empirical formulas, you will need to round each value to its nearest whole number.[7]
-
Example: For the ratio determined in the previous step:
- 2.000 Na can be written as 2 Na.
- 2.012 S can be rounded down to 2 S.
- 2.904 O can be rounded up to 3 O.
-
Example: For the ratio determined in the previous step:
-
7Write your final answer. Translate the ratio of elements into the standard format used for empirical formula. The molecular amount of each element should be indicated in subscript beside its respective element's symbol for all amounts greater than one.
- Example: For a compound that is 2 parts Na, 2 parts S, and 3 parts O, the empirical formula should be written as: Na2S2O3
Method Two: Using Weight in Grams
-
1Consider the number of grams. If you are given the elemental composition of an unknown substance in grams, you will need to proceed according to the following instructions.[8]
- On the other hand, if you are given the composition in percentages instead of grams, see the instructions on "Using Weight Percentages."
- Example: Determine the empirical formula of an unknown substance made from 8.5 g Fe (iron) and 3.8 g O (oxygen.)
-
2Convert the mass of each element into moles. To determine the molecular ratio of elements in the compound, you need to convert the amount of each element from grams to moles. Do so by dividing the mass in grams for each element by the elements' respective atomic weights.[9]
- From a more technical perspective, you are actually multiplying the mass in grams by the mole ratio per atomic weight.
- Note that the atomic weight should be rounded to four significant places to maintain a certain degree of accuracy in your calculations.
-
Example: When there are 8.5 g Fe and 3.8 g O:
- 8.5 g Fe * (1 mol Fe / 55.85 g Fe) = 0.152 mol Fe
- 3.8 g O * (1 mol O / 16.00 g O) = 0.238 mol O
-
3Divide each mole value by the smallest calculated number. Determine how much of each element is present when compared to the other elements in the compound. In order to calculate this, you will need to identify the smallest number of moles present and divide each number of moles by that number.[10]
-
Example: For this problem, the smallest amount of moles present is 0.152 moles (the amount of Fe, iron, present).
- 0.152 mol Fe / 0.152 mol = 1.000 Fe
- 0.238 mol O / 0.152 mol = 1.566 O
-
Example: For this problem, the smallest amount of moles present is 0.152 moles (the amount of Fe, iron, present).
-
4Multiply the ratio values to find near whole numbers. Oftentimes, the moles present for each substance may not equal a whole number. If the excess is within one-tenth, you can simply round it off. For excess values that exceed this, however, you will need to multiply each value by a number that can bring the ratio value nearer to a whole number.
- For instance, if one element has an excess near 0.25, multiply each element amount by 4. If an element has an excess near 0.5, multiply each element amount by 2.
-
Example: Since the ratio amount of oxygen equals 1.566, you will need to multiply both ratio amounts by 2.
- 1.000 Fe * 2 = 2.000 Fe
- 1.566 O * 2 = 3.132 O
-
5Round your answer to the nearest whole number. Once the ratio values of all elements in the compound are roughly within one-tenth of a whole number, you can round off any difference to the nearest whole number.
- Example: The amount of Fe can be written as 2. The amount of O can be rounded down to 3.
-
6Write the final answer. The ratio of elements should be rewritten in empirical formula form.[11] Each ratio value should be indicated in subscript beside its respective element's symbol, unless the ratio value equals one.
- Example: For a compound that is 2 parts Fe and 3 parts O, the empirical formula is: Fe2O3
Method Three: Using Molecular Formula
-
1Determine if the subscripts can be reduced. If you have the molecular formula of an unknown compound but are told to identify the compound by its empirical formula, you need to determine if the formula can be reduced. Look at the subscripts for each element present. If all three subscripts share at least one common factor (other than the number 1), you will need to take a few more steps to determine the compound's empirical formula.[12]
- Example: C8H16O8
- On the other hand, if the subscripts do not all share a common factor, the molecular formula is also the empirical formula.
- Example: Fe3O2H7
-
2Find the greatest common factor between the subscripts. Write the factors of each subscript within your formula. Identify which factor is greatest in value.
-
Example: For C8H16O8, the subscripts are "16" and "8."
- The factors of 8 are: 1, 2, 4, 8
- The factors of 16 are: 1, 2, 4, 8, 16
- The greatest common factor (GCF) between the two numbers is 8.
-
Example: For C8H16O8, the subscripts are "16" and "8."
-
3Divide each subscript by the greatest common factor. To get each subscript in its simplest form, you will need to divide all subscripts present in the formula by the GCF you just found.[13]
-
Example: For C8H16O8:
- Divide the subscript of 8 by the GCF of 8: 8 / 8 = 1
- Divide the subscript of 16 by the GCF of 8: 16 / 8 = 2
-
Example: For C8H16O8:
-
4Write the final answer. Replace your original subscripts with their simplified values. In doing so, you have determined the compound's empirical formula from its molecular formula.
- Note that values of 1 are not usually indicated with subscripts.
- Example: C8H16O8 = CH2O
Community Q&A
-
QuestionHow do I convert grams into moles?
 Community AnswerBy using the molecular mass (sum of the atomic (molar) masses on the periodic table). m/n = M with n = numer of moles; m = mass in grams (g); and M = molecular mass of the compound in grams/moles.
Community AnswerBy using the molecular mass (sum of the atomic (molar) masses on the periodic table). m/n = M with n = numer of moles; m = mass in grams (g); and M = molecular mass of the compound in grams/moles. -
QuestionWhat if the weight of the unknown compound is 500 g/mol? Should the sum of each element equal to 500g/mol?
 KnowledgeIsForeverCommunity AnswerNo. If you count all the elements' molecular weights together (multiplied by how often the compound contains it), the result should be 500 g/mol.
KnowledgeIsForeverCommunity AnswerNo. If you count all the elements' molecular weights together (multiplied by how often the compound contains it), the result should be 500 g/mol.
References
- ↑ https://chem.libretexts.org/Courses/Eastern_Wyoming_College/EWC%3A_Introductory_Chemistry_(Budhi)/06%3A_Chemical_Composition/6.8%3A_Calculating_Empirical_Formulas_for_Compounds
- ↑ https://chem.libretexts.org/Courses/Eastern_Wyoming_College/EWC%3A_Introductory_Chemistry_(Budhi)/06%3A_Chemical_Composition/6.8%3A_Calculating_Empirical_Formulas_for_Compounds
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/06%3A_Chemical_Composition/6.08%3A_Calculating_Empirical_Formulas_for_Compounds
- ↑ https://openstax.org/books/chemistry-2e/pages/3-2-determining-empirical-and-molecular-formulas
- ↑ https://openstax.org/books/chemistry-2e/pages/3-2-determining-empirical-and-molecular-formulas
- ↑ https://openstax.org/books/chemistry-2e/pages/3-2-determining-empirical-and-molecular-formulas
- ↑ https://sccollege.edu/Departments/STEM/Questions/Wiki%20Pages/Empirical%20Formula.aspx
- ↑ https://www.chemteam.info/Mole/Emp-formula-given-percent-comp.html
- ↑ https://www.chemteam.info/Mole/Emp-formula-given-percent-comp.html
- ↑ https://www.chemteam.info/Mole/Emp-formula-given-percent-comp.html
- ↑ https://www.chemteam.info/Mole/Emp-formula-given-percent-comp.html
- ↑ http://chemcollective.org/activities/tutorials/stoich/ef_molecular
- ↑ https://pressbooks.bccampus.ca/chem1114langaracollege/chapter/3-2-determining-empirical-and-molecular-formulas/
About This Article
To determine an empirical formula using weight percentages, start by converting the percentage to grams. For example, if your empirical formula contains 29.3 percent sodium, convert it to 29.3 grams. Next, convert the grams to moles by dividing 29.3 grams by the atomic weight of sodium, which is 22.99 grams, to get 1.274. Then, divide each element’s moles by the smallest number of moles in the formula to find their relative weights. Finally, multiply all the moles by the same number to get whole numbers rather than fractions. To learn more, like how to determine an empirical formula using the molecular formula, read on!