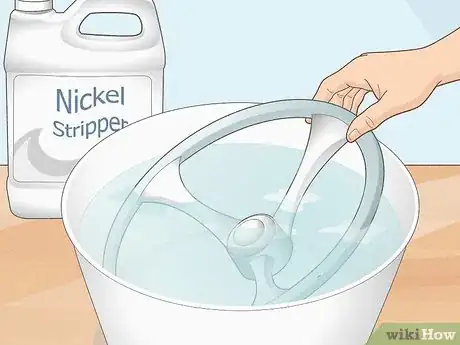This article was co-authored by wikiHow staff writer, Eric McClure. Eric McClure is an editing fellow at wikiHow where he has been editing, researching, and creating content since 2019. A former educator and poet, his work has appeared in Carcinogenic Poetry, Shot Glass Journal, Prairie Margins, and The Rusty Nail. His digital chapbook, The Internet, was also published in TL;DR Magazine. He was the winner of the Paul Carroll award for outstanding achievement in creative writing in 2014, and he was a featured reader at the Poetry Foundation’s Open Door Reading Series in 2015. Eric holds a BA in English from the University of Illinois at Chicago, and an MEd in secondary education from DePaul University.
There are 14 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 47,159 times.
Learn more...
Nickel plating refers to any process that covers a metal object in a protective layer of nickel or nickel alloy. If you’re interested in doing this at home, your only realistic option is nickel electroplating, although there are services out there that offer alternative nickel-plating methods as well. There are two main reasons to nickel-plate an object—to protect it, or improve its overall appearance. Whether you want to breathe new life into that antique jewelry or you’re looking to protect the lug nuts on your vintage bike, read on to learn more about the nickel plating process.
Steps
Can you nickel-plate at home?
-
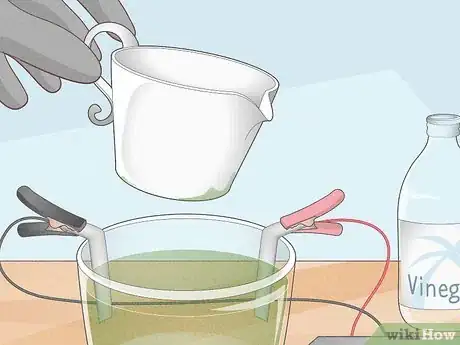
You can, although you do need to wear gloves and protective eyewear. All you need is 2 pieces of nickel anode, which you can buy online. You’ll also need some white vinegar and alligator clips for a battery or power source. Make sure that you keep the room ventilated by opening the windows and turning the fan on. Wear rubber gloves, a dust mask, and protective eyewear.[1] X Research source
- This process is known as electroplating, and it’s the only DIY method you can use to nickel-plate an object. There are other, more effective methods when it comes to nickel plating, but you’ll need to pay someone to do this.
How do I set up my nickel plating equipment?
-
Fill a glass container with vinegar and a little salt. Hang your two pieces of nickel on the rim of the container so that they’re halfway submerged. Grab a small power supply and use the alligator clips to hook the positive and negative leads up to the pieces of nickel. Hook the positive wire up to one of the nickel pieces, and the negative wire to the other one. Plug the power supply in and wait.[2] X Research source
- For your power supply, you can use a 6- or 12-volt battery. You can also buy a dedicated power supply with built-in alligator clips. If you’re feeling really crafty, you can use an old phone charger by splitting the cable, separating the two insulated wires, and hooking up to that.[3] X Research source
- So long as the power supply isn’t putting out more than 1 amp, you’ll be fine.[4] X Research source
How do I nickel-plate my object?
-
Soak it in the solution once the vinegar turns green. The salt, vinegar, and charged nickel anodes will flood the solution with electrolytes and cause it to turn green. Once this happens, it is charged. Clean the metal object you want to plate and disconnect the power supply. Leave the positive nickel piece hooked up where it is, but take the negative off. Hang your object from some copper wire and submerge it in your solution.[5] X Research source
- It should take roughly 20 minutes for the nickel plating to be complete.
- Hanging your object in the solution will keep it from floating to the bottom of the container. If this happens, the plating won’t coat each side of your object evenly.
Does nickel plating wear off?
-
Yes, the plating will wear away over time. However, it usually takes a long time and a heavy amount of wear and tear. There are two main benefits when it comes to nickel plating. One is that it generally looks pretty cool. The other is that it protects the underlying material from damage. So, while the nickel plating will eventually fade, it will take a long time and it’s a sign that the plating is doing its job by protecting the surface underneath.[6] X Research source
Is nickel plating expensive?
-
It totally depends on the object and your preferred method. There are a few different ways to nickel-plate an object, and a computer part requires a different level of attention to detail than a lug nut. Luckily, most plating services offer free quotes. Contact a few different services near you to see what they’ll charge based on what you’re looking to nickel-plate.[7] X Research source
- If you’re doing a DIY electroplating bath, all you need to buy are two pieces of nickel anode. You may also need to buy two alligator clips, but it shouldn’t cost you more than a couple of dollars all things considered.
How can you tell if nickel is plated?
-
Scratch the object and submerge it in saltwater for 24 hours. Nickel-plated objects will corrode in the salt water, and their color will change dramatically. Authentic 100% nickel won’t corrode or change color.[8] X Research source
- If you don’t want to risk damaging the item, you could always take the object to a jeweler and ask them to take a look at it. They should be able to determine if you have pure nickel or not.[9] X Research source
What color is nickel plating?
-
If you do this at home, it will be silver, just like regular nickel. If the process wasn’t carried out 100% correctly, your nickel may have a slightly yellow tinge. The type of plating influences the texture, but not the color. Your DIY nickel plating will come out bright and shiny. Other professional methods may leave the plating with a duller or matte finish.[10] X Research source
- Professional nickel-plating services can add dyes to the nickel coating. These coatings can be any color.[11] X Research source
Is nickel toxic to touch?
-
No, although it may be toxic if you’re allergic to nickel. If you are allergic to nickel and you touch something plated, you may develop contact dermatitis. Your skin may develop a rash, feel itchy, or turn red and dry. These symptoms usually go away on their own, but you should see a doctor if the symptoms get worse over time.[12] X Trustworthy Source Mayo Clinic Educational website from one of the world's leading hospitals Go to source
- Nickel is actually found, in trace amounts, in the air, water, and various household products. So long as you aren’t swallowing it or actively rubbing nickel all over your body, you should be fine.[13] X Research source
Is nickel plating better than chrome?
-
Go for nickel plating if you want protection and chrome for the look. If your object is going to be exposed to a lot of other chemicals or moisture, then you’re better off doing nickel plating. Chrome is a slightly harder material, but nickel is close enough in that race that you won’t see a big difference there. The main reason to select chrome plating is that it looks neat. It’s shinier, more reflective, and has this neat blue tint when you shine a light on it.[14] X Research source
- It is generally more expensive to get chrome plating, although it depends on the type of nickel plating you go for.
How do you remove nickel plating?
-
Buy a nontoxic nickel stripping agent and soak your object. Rinse your object off. Follow the instructions on your nickel plating stripping agent to heat it up. Typically, you heat the liquid up to around 140 °F (60 °C) to activate it. Then, soak your nickel-plated object in the solution for 10-15 minutes. Remove it with some tongs or a wooden spoon, rinse the object off thoroughly, and you’re done![15] X Research source
- You can buy a nickel stripping agent online.
- There are other ways to do this, but it usually involves charging sulfuric acid with anodes, and it’s a little more complicated and dangerous than just using a stripping agent.[16] X Research source
You Might Also Like
 Building and Tuning a Wind Chime at Home
Building and Tuning a Wind Chime at Home







References
- ↑ https://nickelinstitute.org/media/2323/nph_141015.pdf
- ↑ https://youtu.be/G-PtnwtOR24?t=46
- ↑ https://youtu.be/G-PtnwtOR24?t=12
- ↑ https://youtu.be/G-PtnwtOR24?t=12
- ↑ https://youtu.be/G-PtnwtOR24?t=287
- ↑ https://www.explainthatstuff.com/electroplating.html
- ↑ https://www.sharrettsplating.com/plating-methods/electroless-nickel-plating-cost
- ↑ https://youtu.be/w40t0RxQd3Q?t=4
- ↑ https://www.thermofisher.com/blog/metals/seth-gold-tells-all-national-pawnbroker-of-the-year-explains-the-need-to-drop-the-acid/
- ↑ https://www.dorsetware.com/types-nickel-plating/
- ↑ https://nickelinstitute.org/media/2323/nph_141015.pdf
- ↑ https://www.mayoclinic.org/diseases-conditions/nickel-allergy/symptoms-causes/syc-20351529
- ↑ https://sites.dartmouth.edu/toxmetal/more-metals/nickel-hidden-in-plain-sight/the-facts-on-nickel/
- ↑ https://www.pfiinc.com/hard-chrome-plating-vs-electroless-nickel-plating-the-differences-explained/
- ↑ https://youtu.be/UcFZJNs29X4?t=27
- ↑ https://youtu.be/INiyYbFUihA?t=25
About This Article