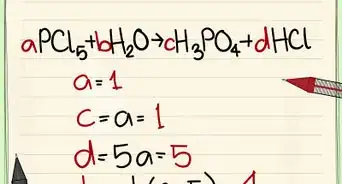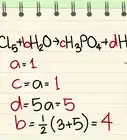X
wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 9 people, some anonymous, worked to edit and improve it over time.
This article has been viewed 49,531 times.
Learn more...
This is the best and simplest guide to naming compounds with hydrogen and carbon in them! Don't be put off by the long and complicated names, start from the basics.
Steps
-
1See how many Carbon atoms are in the chain.
-
2Look at the table to determine what its name is. For 'A level' you must memorize all of names in this table.[1]Advertisement
-
3Note the other variant. The other variation of the hydrocarbon is to have a double bond in them, like this. Replace the -ane with an -ene. For example this would be Ethene.[2]
-
4Examine the Haloalkane! The halo- in its name comes from Halogen.
- Learn how to name them from reading the next steps.
-
5Number the carbons, starting from the Halogen (or functional group).
-
6Use this table. You should find the name you will add to your chain's name.
-
7Name it. Keep in mind that the "-" is essential for naming compounds. It must always be between a number and a letter, like this "2-chloro...."[3]
-
8Know what a functional group! This is a functional group.[4]
-
9Name a function group. To do so, make sure to count from the side that gives it the smallest number. In this case it can only be 2.
-
10
-
11Introduce two Halogens into the hydrocarbon.
-
12Count out on which number carbon the Halogens are attached to. Remember to keep the lowest numbered carbons.
-
13Remember another essential piece of information.
-
14Get the Halogens in alphabetical order. Example: Bromo comes before Chloro. Use this information here is for the name of this molecule.[5]
Advertisement
Community Q&A
-
QuestionThat is just for hydrocarbons right? What about other organic compounds?
 Community AnswerOrganic compounds are defined by the presence of hydrogen-carbon structures. While hydrocarbons often refer to burnable compounds, the words mean pretty much the same thing. Naming complex organic compounds such as codeine or adenosine is difficult and will not be expected from high school or first year university students.
Community AnswerOrganic compounds are defined by the presence of hydrogen-carbon structures. While hydrocarbons often refer to burnable compounds, the words mean pretty much the same thing. Naming complex organic compounds such as codeine or adenosine is difficult and will not be expected from high school or first year university students. -
QuestionHow many lone pairs does ammonia have?
 Community AnswerThe molecular formula of ammonia is NH3. The nitrogen has 5 number of valance electrons; it is bonded to 3 hydrogen atoms, so it has 1 lone pair of electrons.
Community AnswerThe molecular formula of ammonia is NH3. The nitrogen has 5 number of valance electrons; it is bonded to 3 hydrogen atoms, so it has 1 lone pair of electrons. -
QuestionIs coca cola a compound or mixture?
 HannahCommunity AnswerIt is a mixture of water, sugar, coloring, flavoring agents, acids and many more. A compound would be a pure substance, which means it's made up out of only one kind of molecule - like water, which consists only of H2O.
HannahCommunity AnswerIt is a mixture of water, sugar, coloring, flavoring agents, acids and many more. A compound would be a pure substance, which means it's made up out of only one kind of molecule - like water, which consists only of H2O.
Advertisement
References
- ↑ https://www.chemguide.co.uk/basicorg/conventions/names.html
- ↑ https://www.chemguide.co.uk/basicorg/conventions/names.html
- ↑ https://www.acdlabs.com/iupac/nomenclature/93/r93_45.htm
- ↑ https://www.siyavula.com/read/science/grade-12/organic-molecules/04-organic-molecules-03
- ↑ https://www.siyavula.com/read/science/grade-12/organic-molecules/04-organic-molecules-03
About This Article
Advertisement
-Step-1-Version-2.webp)
-Step-2-Version-2.webp)
-Step-3-Version-2.webp)
-Step-4.webp)
-Step-5-Version-2.webp)
-Step-6-Version-2.webp)
-Step-7-Version-2.webp)
-Step-8-Version-2.webp)
-Step-9-Version-2.webp)
-Step-10-Version-2.webp)
-Step-11-Version-2.webp)
-Step-12.webp)
-Step-13-Version-2.webp)
-Step-14.webp)
-Step-15.webp)