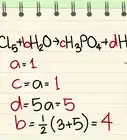This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
This article has been viewed 35,332 times.
Citric acid (C6H8O7) is a weak organic acid present in oranges, lemons, limes and other citrus fruits. It is also commonly found in the lab and/or kitchen. You can use this acid in your cooking and cleaning, or to carry out experiments in the lab. Sometimes, you will need to dilute and neutralize citric acid (e.g., before disposing of it in lab). When this happens, you will need to understand and follow safe procedures for diluting the citric acid and neutralizing the solution.
Steps
Diluting Citric Acid
-
1Fill a container with water. Diluting a substance simply means to make it less concentrated. In this instance, you will be increasing the amount of water in the solution, thus diluting the acid. The first step is to have your water ready to receive the acid.[1]
- Do not pour the water into the acid. Put the water in a separate container.
-
2Pour the acid into the water. Once you have water ready for dilution, you can slowly pour the citric acid into the water. If you have a large volume of citric acid, pour a little and then stir the solution before pouring more. This will help disperse the heat that is generated when diluting the acid.[2]
- Never pour water into acid. The heat will not be well dispersed, and it can result in concentrated acid vaporizing or splashing onto you.
- Wear gloves and goggles when diluting acid.
Advertisement -
3Monitor the pH of the solution. The pH of the dilute acid solution will be higher than that of the concentrated acid solution. It should be noted that the pH of your dilute solution will never raise above 7.0 because that is the pH of the water. The closer you can bring the pH to 7.0 (neutral), the safer it will be to neutralize the acid with a base.[3]
Reacting Citric Acid with a Base
-
1Choose a base. Bases are substances with a high pH. Like acids, bases are corrosive. However, when acids and bases contact each other, they neutralize each other and the resulting mixture is less corrosive. You can use a strong base like NaOH (sodium hydroxide) to neutralize citric acid. If you do not have access to NaOH, something like sodium bicarbonate (baking soda) will also neutralize citric acid well.
-
2Dilute the base. Dilute your base the same way that you diluted the citric acid. Get a container of water and slowly mix in the base. Again, the closer you bring the pH to 7.0, the safer the reaction will be when you use it to neutralize the citric acid solution.[4]
- Note that 7.0 is as low as you can get when diluting a base in water. This is because the pH of the water is 7.0, and thus adding more water will not make the pH go lower.
- Wear gloves and goggles when diluting bases.
-
3Pour the acid solution into the base. To neutralize the citric acid solution, pour it slowly into the basic solution. Be prepared for the reaction to fizz and get hot. The reaction is also likely to emit fumes. Avoid getting any acid or base on your skin, and do not inhale any fumes produced. Just continue adding the acid slowly and stirring when needed.[5]
- The fizz is caused by the formation of carbon dioxide gas bubbles during the reaction.
- Use a glass stir rod to stir the reaction.
- Wear gloves and goggles when reacting acids and bases.
-
4
Understanding Acid Base Chemistry
-
1Know the difference between acids and bases. If you are looking to neutralize acids like citric acid, you should know what they are. Acids are compounds that easily give up a proton (hydrogen ion). As such, they are called proton donors. Bases are compounds that readily accept extra protons and are called proton acceptors. Acids and bases neutralize each other because a base can accept the acid’s extra protons, which usually results in the formation of water, salt, and sometimes a gas such as CO2 (depending on which acids and bases you react, the products will be different).
-
2Realize that water is amphoteric. Many acid and base reactions are done in water. This is an important fact since water can act as either an acid or a base. That is, the H2O molecule can donate a proton and become a hydroxide ion (OH-) or it can accept an extra proton and become a hydronium ion (H3O+). The ability to function as an acid and a base is referred to as amphoterism.
-
3Understand common byproducts of acid/base reactions. Because acids and bases form ions, they will also form salts when they react. Strong acids and bases dissociate completely in water and usually form only salt and water when mixed. Weak acids and bases only dissociate partially. That means that there may be other byproducts, such as carbon dioxide gas, when they are reacted.
- An example of a strong acid and strong base mixing is NaOH (sodium hydroxide) and HCl (hydrochloric acid). When they mix, the salt NaCl is formed along with water. No other byproducts are formed.
- A common example of a weak acid and base reaction is the one that occurs between citric acid and baking soda. The reaction forms salt, water, and carbon dioxide.
Expert Q&A
-
QuestionWhat happens when you add phosphoric acid to citric acid?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher Phosphoric acid (H3PO4) is also a weak organic acid like citric acid. Combining the two yields cyclohexane, water, and dihydrogen phosphate (the conjugate base of phosphoric acid). At some concentrations, phosphoric acid can be corrosive. Also, adding an acid to another acid will not neutralize the solution and could make it more acidic and/or dangerous to handle in the kitchen or the lab.
Phosphoric acid (H3PO4) is also a weak organic acid like citric acid. Combining the two yields cyclohexane, water, and dihydrogen phosphate (the conjugate base of phosphoric acid). At some concentrations, phosphoric acid can be corrosive. Also, adding an acid to another acid will not neutralize the solution and could make it more acidic and/or dangerous to handle in the kitchen or the lab.
Warnings
- Acids and bases are corrosive and can be dangerous. You should always wear gloves when handling either one. If you are using strong acids and bases, it’s a good idea to wear protective goggles, as well.⧼thumbs_response⧽
References
- ↑ http://www.physics-chemistry-class.com/chemistry/dilution-acid-base.html
- ↑ http://www.physics-chemistry-class.com/chemistry/dilution-acid-base.html
- ↑ http://www.physics-chemistry-class.com/chemistry/dilution-acid-base.html
- ↑ http://www.physics-chemistry-class.com/chemistry/dilution-acid-base.html
- ↑ http://www.physics-chemistry-class.com/chemistry/dilution-acid-base.html
- ↑ http://www.physics-chemistry-class.com/chemistry/dilution-acid-base.html