This article was co-authored by Chris Hasegawa, PhD. Dr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
There are 10 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, several readers have written to tell us that this article was helpful to them, earning it our reader-approved status.
This article has been viewed 161,529 times.
Organic Chemistry has a nasty reputation — it's not uncommon for students to hear horror stories about the class's difficulty before they have a chance to take it themselves. While the class can be a challenging one, "O Chem" isn't the nightmare it's often cracked up to be. There is less information to memorize but more processes to understand, so an understanding of the fundamentals and a good study regimen are key to getting a passing grade.
Steps
Basic O Chem Knowledge
-
1Learn the definition of "organic chemistry." Generally speaking, organic chemistry is the study of carbon-based chemical compounds.[1] Carbon is the sixth element in the periodic table and one of the most vital building blocks for life on earth. Living things are made up of molecules made mainly of carbon. This means that O chem includes the chemistry that goes on inside your body every day. It also includes the chemistry that occurs inside animals, plants, and natural ecosystems.
- However, organic chemistry isn't limited to living things. For example, the chemical reactions involved in the burning of fossil fuels fall under the O chem umbrella because these reactions involve carbon-based compounds in the fuels.
-
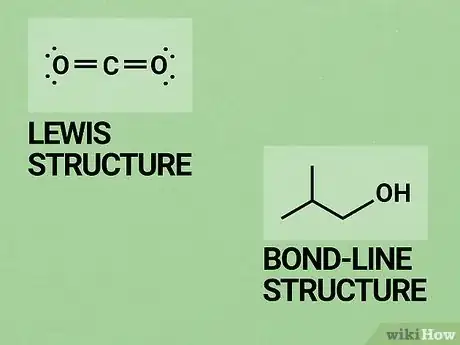
2Learn the common ways of representing molecules. O chem is a more "visual" area of study than general chemistry. You will be relying on drawings of molecules and compounds more often than in previous chemistry classes. Knowing how to interpret these sorts of visual representations is one of the most basic, important O chem skills.
- You'll need to be familiar with Lewis structures before you begin. These are typically taught as part of a general chemistry course. In a Lewis structure, atoms in a molecule are represented by their chemical symbol (their letters on the periodic table). Lines represent the bonds between them and dots represent their valence electrons. See our Lewis structure article for a refresher.
- One way of drawing molecules that will probably be new to you is the skeletal formula method. In a skeletal formula (also called "bond-line structure"), carbon atoms are not shown. Instead, there is just a line to represent the bond. Since there are so many carbon atoms in O chem, this makes it much quicker to draw molecules. Non-carbon atoms are still represented by their chemical symbols. A good guide to skeletal formations is available here.[2]
Advertisement -
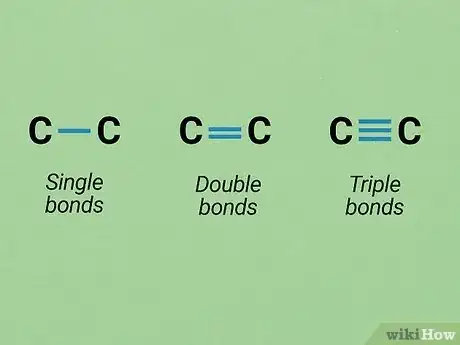
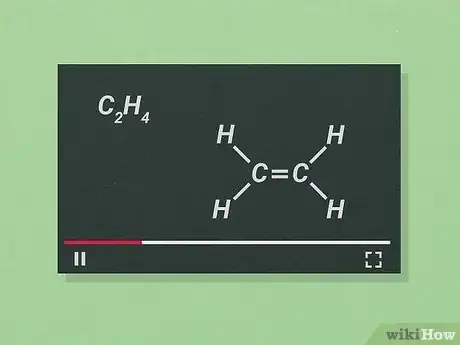
3Learn how to represent bonds. Covalent bonds are by far the most common type of bonds you will be dealing with in O chem (though knowledge of ionic bonds, etc. is still valuable). In a covalent bond, two atoms share unpaired electrons to form a bond. If extra unpaired electrons are available, double- or triple-bonded atoms are possible.
- In both Lewis structures and skeletal formulas, single bonds are represented by one line, double lines by a double line, and triple bonds by a triple line.
- In skeletal formulas, the bonds between carbon (C) and hydrogen (H) atoms are not usually drawn because they are so common.
- Except in special circumstances, atoms are typically only allowed to have eight valence (outer shell) electrons. This means that most of the time an atom can bond to a maximum of four other atoms.
-
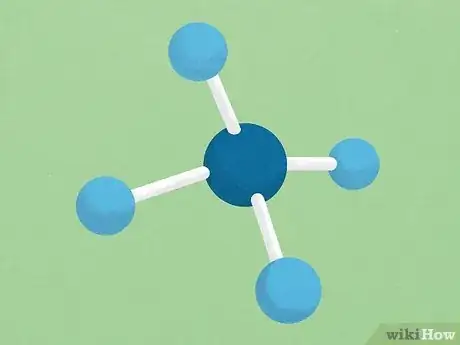
4Learn the basics of 3-D molecular structure. Organic chemistry requires students to think about chemical molecules the way they actually exist in real life — not just the way they are drawn. Molecules take the form of three-dimensional structures. The nature of the bonds in the molecule is the most important thing for determining the 3-D shape of a molecule, though other factors can sometimes also play a role. Below are a few things to remember when it comes to the shapes of carbon-based molecules:
- A carbon bonded to four other atoms with single bonds will have the shape of a tetrahedron (four-pointed pyramid). A good example of this is the molecule methane (CH4)
- A carbon bonded to another atom with a double bond and two atoms with single bonds has a trigonal planar (flat triangle) shape. The ion CO3-2 is a good example here.
- A carbon bonded to two atoms with double bonds or bonded to one album with a triple bond has a linear (rigid line) shape. The molecule carbon dioxide (CO2) is one example.
-
5Learn to decipher orbital hybridization. This topic has an intimidating name, but it's not that hard to understand. Essentially, hybrid orbitals are just ways that chemists represent an atom's valence electrons based on how that atom behaves (rather how it is drawn). If an atom has a certain number of unpaired electrons available for bonding but tends to form a different number of bonds, it is said that it has "hybrid orbitals" to make up the difference.
- Carbon is a perfect example of this. Carbon atoms have four valence electrons: two in the 2s orbital and two unpaired ones in the 2p orbital. Since there are two unpaired electrons, it might be expected that carbon will form two bonds. However, experiments show that the paired electrons in the 2s orbital form bonds even though they are not unpaired. Thus, we say that the carbon atom has four unpaired electrons in an sp hybrid orbital.
-
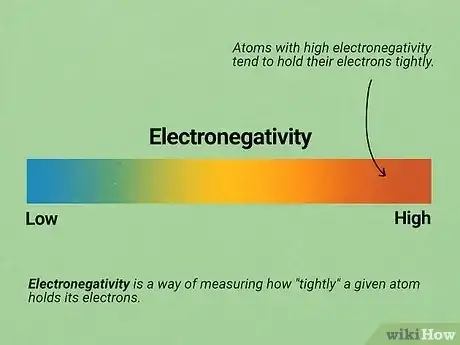
6Learn the basics of electronegativity. There are many, many factors that can determine how two molecules react with each other in O chem. However, electronegativity is often one of the most important factors. Electronegativity is a way of measuring how "tightly" a given atom holds its electrons. Atoms with high electronegativity tend to hold their electrons tightly (and vice versa for atoms with low electronegativity). See our electronegativity article for detailed information.
- As you go up and to the right in the periodic table, atoms gain more electronegativity (hydrogen and helium are not included). Fluorine, the atom in the very top top right, has the highest electronegativity of all.
- Because electronegative atoms "want" more electrons, they tend to react by "grabbing" available electrons on other molecules. For example, atoms like chlorine and fluorine often appear as negative ions because they have taken electrons from other atoms.[3]
Study Tips
-
1Don't be intimidated. Organic chemistry introduces a lot of new concepts and forces you to think about chemistry problems in new ways. You will have to learn a whole new chemistry "vocabulary." Relax — everyone in your class is facing the same challenges. Study diligently and get the help you need and you will likely be fine.
- Organic chemistry isn't hard—you just have to reach a point where you can easily visualize it.[4]
- Don't let horror stories from people who have taken O chem before get to you.[5] Students tend to embellish how difficult their experiences were. Going into your first test terrified that you are facing an impossible challenge will only make things harder.[6] Instead, boost your confidence by spending plenty of time studying and getting plenty of rest the night before.
- O chem is not a math-heavy course. You will not have to do much arithmetic or algebra to pass this course. Rather, think of the subject more like learning a new language.[7]
-
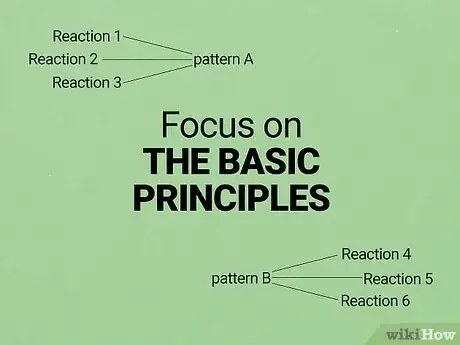
2Focus on understanding rather than memorization. You will likely see hundreds of different reactions in your O chem class. It's virtually impossible to memorize them all, so don't waste your time memorizing any but the most important. Instead, focus on the basic principles behind the most common reactions. Most reactions follow one of just a few patterns, so understanding these patterns well and knowing how to apply them is a much more efficient way to get problems right.
- However, if you are good at memorization, you can still use this to your advantage. Try writing basic reaction mechanisms on flashcards and using these to memorize the reactions. You'll still need to be able to adjust your knowledge when you see reactions that you're not familiar with, but you can use the basic principles to guide you towards the correct mechanism.
- Repetition is a great way to better understand organic chemistry. As you study, grab a whiteboard and draw things over and over again.[8]
-
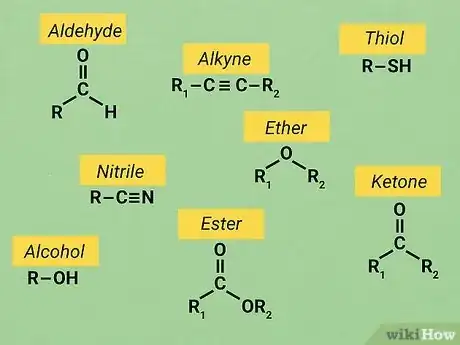
3Know your functional groups. Basic O chem uses the same set of reactive structures in nearly all of its molecules. These structures are known as "functional groups." Knowing how to identify these functional groups as well as how they tend to react it crucial for many O chem assignments. Since functional groups usually react in the same way with consistency, knowing their traits allows you to solve a wide variety of problems.
- There are too many functional groups in organic chemistry to list in this article. However, it's not hard to find guides to the functional groups online. For example, a good guide from Purdue University is available here.
-
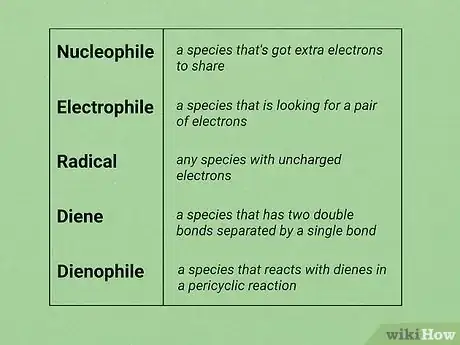
4Learn the concepts of nucleophile and electrophile. Most organic reactions fall into three types: nucleophile/electrophile, pericyclic, and radical, and by far the most common are nucleophile/electrophile reactions. Some important classes of reagents you will need to know about are:
- Nucleophile: a species that's got extra electrons to share. Look out for negatively charged species, double bonds, or neutral species with lone pairs. Examples include hydroxide, pyridine, iodide, alkenes, enolates, and Grignard reagents.
- Electrophile: a species that is looking for a pair of electrons. Look out for partial or fully positively charged species. Examples include carbocations, hydrogen halide acids, haloalkanes, hydronium ions, and carbonyls.
- The diatomic halogens (Cl2, Br2) do not bear positive charges, but the bond between the two halogen atoms is weak and they are able to make stable anions, rendering them susceptible to nucleophilic attack.
- Radical: any species with uncharged electrons. This could include a bromine atom, for example. When these species react, they tend to make one "normal" molecule, along with another radical.
- Diene: a species that has two double bonds separated by a single bond (conjugated double bonds); these participate in pericyclic reactions. Common compounds of this type include furan, Cyclopentadiene, and 1,3-butadiene.
- Dienophile: a species that reacts with dienes in a pericyclic reaction. Look out for an alkene conjugated to a carbonyl group (an α,β-unsaturated carbonyl compound) such as ethyl acrylate, methyl vinyl ketone, or cyanoacrylate.
-
5When in doubt, follow the flow of electrons. At their most basic level, most organic chemistry reactions just involve two or more molecules exchanging electrons. If you can't figure out how to get started on a reaction mechanism, start by looking for places where it makes sense for electrons to transfer. In other words, look for atoms that seem like especially good electron acceptors and atoms that look like especially good electron donors. Make the transfer and then ask, "what do I need to do now to get my new molecules to a stable state?"
- As one example, since oxygen (O) is more electronegative than carbon, the O that is double-bonded to C in a ketone group tends to hold the electrons in the bond closer to itself. This gives C a partially positive charge and makes it a good candidate to receive electrons. If you have a good electron donor involved in the reaction, it makes sense that it might attack the C, forming a new bond and kicking off your reaction.
-
6Use study groups for homework and tests. Don't ever feel like you need to tackle organic chemistry by yourself. Getting together to do your work with your fellow students is a fantastic idea. Not only can others help you with concepts you're struggling with — you can also get a stronger grasp on material you already know by explaining it to someone else.
Getting Help
-
1Get to know your professor. The person with the best knowledge of O chem in your classroom is the person teaching the class. Take advantage of this valuable resource. Visit your teacher's office to discuss areas you are having trouble with. Try to have a few clear, succinct questions to ask or a problem or two that you are having trouble with. Be ready to explain the thought process that got you to the wrong answer.
- Avoid walking into your professor's office without a clear idea of what you want. Simply saying "I don't get the homework" won't get you useful help.
- Not only is this a great way of getting your questions answered — it can also help you get to know your professor. Keep in mind that if you're aiming for grad school, you will need a few academic references in the future. Professors are much more likely to write positively of people that took the time to talk to them.
- If you can't get a hold of your professor, then look around for their research lab and student office. Most professors employ a few graduate students in their labs, and they may be willing to help you with questions.
-
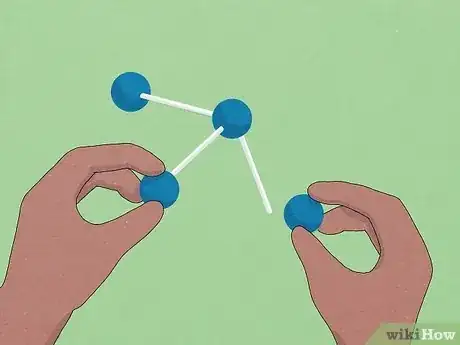
2Use tools to help visualize problems. In O chem, the shapes of molecules can determine how they react. Because it can be difficult to represent complex 3D molecules on a flat piece of paper, using physical building blocks is a great way to wrap your head around difficult structures.
- Molecular model sets allow you to build molecules out of plastic pieces. These can be somewhat expensive if you buy them from your school's book store or a chemical supplier, but some professors will loan them out to students who ask for them at no cost.
- If you can't get your hands on a "real" model set, try using foam balls, markers, and wooden dowels from your local craft store for a DIY alternative.
- Various computer programs (like the one available here) can also help you model molecules in three dimensions.[9]
-
3Join the discussion on help forums. One of the silver linings of O chemistry's high difficulty level is that there are lots of students looking for help online (as well as offering it). Various online chemistry forums have large communities of users willing to discuss difficult organic topics. Try posting a problem that you're having trouble with on one of these forums, then work with the people who respond to get the help you need.
- Though there are many forums for these sorts of problems, chemicalforums.com is a good place to start.[10]
-
4Use online O chem resources. A wide variety of sites can help with difficult O chem topics. Just a few good resources are listed below:
- Khan Academy: Hosts numerous video lectures covering a variety of basic topics.[11]
- Chem Helper: Has links to practice tests, help forums, reaction mechanisms, and more. Also includes laboratory help.[12]
- University of South Carolina Aiken: Includes its own directory of helpful websites covering a variety of O chem topics.
Expert Q&A
-
QuestionHow do you finish a difficult homework problem?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean Take a picture of the homework problem and send it to someone who really understands the material. They can help walk you through whatever you're struggling with.
Take a picture of the homework problem and send it to someone who really understands the material. They can help walk you through whatever you're struggling with. -
QuestionIs organic chemistry more difficult than general chemistry?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean Not necessarily! A lot of students make organic chemistry out to be one of the toughest classes in the world, but it really isn't. You just need to take the time to draw things out so you can visualize them.
Not necessarily! A lot of students make organic chemistry out to be one of the toughest classes in the world, but it really isn't. You just need to take the time to draw things out so you can visualize them. -
QuestionHow can I learn organic chemistry?
 Community AnswerStudying organic chemistry is like studying a language. It has its own alphabet. First study the alphabet (i.e. basics). Then study, write down and now try to solve the problems.
Community AnswerStudying organic chemistry is like studying a language. It has its own alphabet. First study the alphabet (i.e. basics). Then study, write down and now try to solve the problems.
References
- ↑ http://www.acs.org/content/acs/en/careers/college-to-career/areas-of-chemistry/organic-chemistry.html
- ↑ http://www.ivyroses.com/Chemistry/Organic/How-to-draw-skeletal-formulae-of-organic-molecules.php
- ↑ https://www.wyzant.com/resources/lessons/science/chemistry/electronegativity
- ↑ Chris Hasegawa, PhD. Retired Science Professor & Dean. Expert Interview. 29 July 2021.
- ↑ Chris Hasegawa, PhD. Retired Science Professor & Dean. Expert Interview. 29 July 2021.
- ↑ http://www.masterorganicchemistry.com/2011/01/17/how-to-do-well-in-organic-chemistry-one-students-advice/?_ga=1.219393433.1803543740.1435265508
- ↑ https://twitter.com/awhspeed/status/1340281333424254977
- ↑ Chris Hasegawa, PhD. Retired Science Professor & Dean. Expert Interview. 29 July 2021.
- ↑ http://www.chemeddl.org/resources/models360/models.php
About This Article
To pass organic chemistry, focus on understanding the basic principles behind the most common reactions, since most reactions follow one of just a few patterns. Additionally, see if you can get a molecular model set, or find an online molecule modeling program, then use it to help you visualize and solve problems. You might also want to consider forming a study group with classmates, who can help answer your questions and help you solidify your own knowledge as you answer theirs. For tips on how to follow the flow of electrons to figure out what’s going on in a chemical reaction, read on!