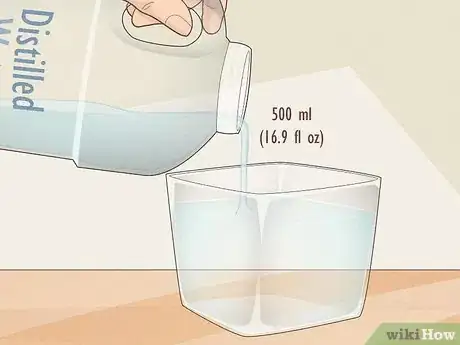X
wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, volunteer authors worked to edit and improve it over time.
This article has been viewed 29,772 times.
Learn more...
If you are a science teacher who wants to amaze your students, or just an average amateur chemist, then this is the experiment for you! The classic iodine clock reaction demonstrates the properties of chemical kinetics through its mesmerizing change in color, and it is sure to fascinate you and perhaps your audience. With just a few household items, you can easily perform this experiment with great success.
Steps
-
1Gather the materials. You will essentially need:
- 10 volume hydrogen peroxide
- 1000 mg vitamin C tablets
- 5% Iodine tincture
- Distilled water
- Cornstarch
- Containers (preferably clear)
- Coffee filters
-
2Prepare the first solution. Crush two of the vitamin C tablets with the back of a spoon until they are nearly powdery. Once that's done, put the powder into a container.Advertisement
-
3Add 60 mL of distilled water to the container with the crushed tablets, and stir for 30 seconds. Don't worry if the powder doesn't completely dissolve — this is expected to happen.
-
4Filter the solution using a coffee filter. It is necessary to get rid of the undissolved particles so that it doesn't interfere with the experiment. Once the filtration is complete, return the liquid to its original container.
-
5Add 25 mL of the iodine tincture to the solution. You will notice that the iodine becomes colorless — this is because the elemental iodine from the tincture breaks up into ions, forming a colorless solution. (If your solution does not turn clear, it means that you didn't put enough vitamin C, and you will need to remake the solution.)
-
6Add water to the solution until the total volume is 500 milliliters (16.9 fl oz). Your first solution is finished!
-
7Prepare the second solution. Add a small amount of cornstarch to another container (around a teaspoon; the exact measurement isn't important).
-
8Add 350 mL of distilled water to the container, mixing it with the cornstarch. Microwave the solution until it boils to dissolve as much cornstarch as possible. The solution may look cloudy, and you can filter it to get rid of the undissolved particles. However, this is optional, and is only done to demonstrate the experiment more clearly.
-
9Add 150 mL of the hydrogen peroxide to the cornstarch solution. Your second solution is now complete.
-
10Begin the reaction! To see the iodine clock reaction in action, mix an equal amount of the first solution and the second solution into a container (you do not need to use all of the solution). Within a minute or two, the liquid should suddenly turn into a dark color. The reaction is quite complex, but what is essentially happening is that the vitamin C is consumed, reverting the iodide ions to iodine molecules, which then react with the starch to form a bluish-black hue.
-
11Have fun with the experiment! Try doing the reaction with different amounts, containers etc. Make some observations: Does the reaction occur faster at higher temperatures? Does the amount of solution used affect the reaction time? Will the mixture change color at the same time if you separated them into two containers?
Advertisement
Community Q&A
-
QuestionWhat percent hydrogen peroxide should be used?
 Community AnswerAccording to online sources, 10 volume hydrogen peroxide is the same as 3% hydrogen peroxide that you buy at the store.
Community AnswerAccording to online sources, 10 volume hydrogen peroxide is the same as 3% hydrogen peroxide that you buy at the store. -
QuestionWhat lf l want to use sodium thiosulphate?
 Community AnswerSodium thiosulfate, also spelled sodium thiosulphate, is used as a medication to treat cyanide poisoning, pityriasis versicolor, and to decrease side effects from cisplatin. For cyanide poisoning it is often used after the medication sodium nitrite and typically only recommended for severe cases. Formula: Na2O3S2.
Community AnswerSodium thiosulfate, also spelled sodium thiosulphate, is used as a medication to treat cyanide poisoning, pityriasis versicolor, and to decrease side effects from cisplatin. For cyanide poisoning it is often used after the medication sodium nitrite and typically only recommended for severe cases. Formula: Na2O3S2. -
QuestionCan small doses of carbon monoxide be used in place of hydrogen peroxide?
 HannahCommunity AnswerNo, it won't work. These two are completely different substances - one is a toxic gas and one is a liquid (at room temperature).
HannahCommunity AnswerNo, it won't work. These two are completely different substances - one is a toxic gas and one is a liquid (at room temperature).
Advertisement
Warnings
- Iodine tinctures can stain clothing and skin, so avoid getting it on them.⧼thumbs_response⧽
Advertisement
About This Article
Advertisement