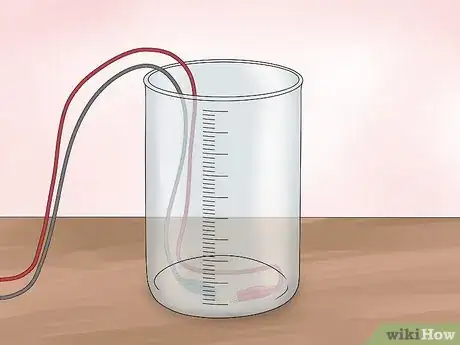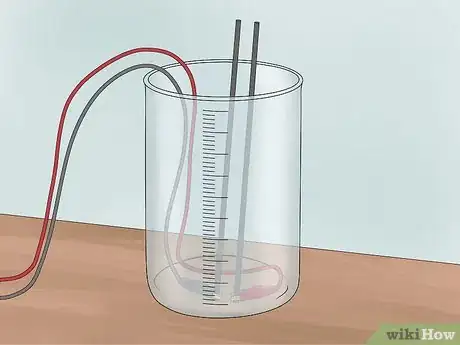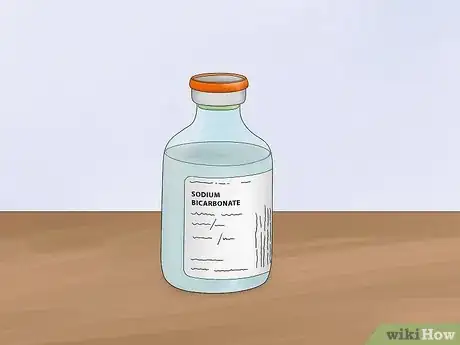This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
This article has been viewed 26,327 times.
You have probably learned somewhere along the way that water is made up of oxygen and hydrogen. Instead of taking someone’s word for it, you can prove this to yourself by separating the water back into its components. The most common technique to separate water molecules is known as electrolysis. Though electrolysis can be done with household supplies, acetic acid (vinegar) does not promote electrolysis enough to generate a noticeable amount of gas. You can prove this to yourself by doing electrolysis with vinegar, and then with baking soda. You will notice that electrolysis with baking soda produces much more gas than with vinegar.
Steps
Building the Apparatus
-
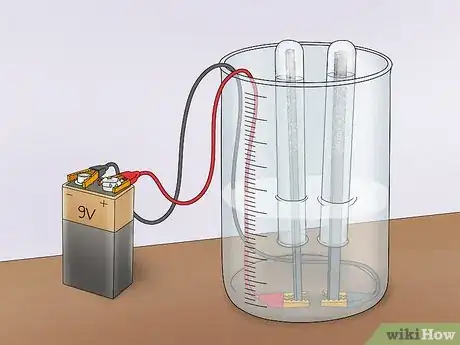
1Start with an empty tub. You can use a plastic or glass tub. Avoid using a metal tub since you will be running an electrical current through the water. The size of the tub does not matter, but a good starting point would be to use a 1⁄2 gallon (1.9 L) container.
-
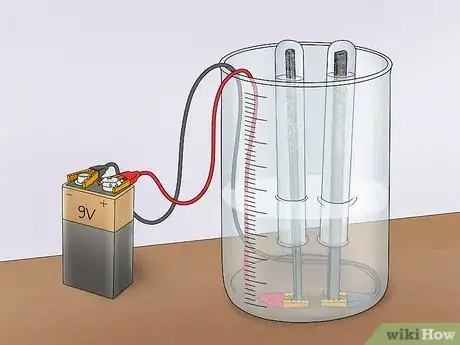
2Tape alligator clips to the bottom of the tub. You will need 2 alligator clips. One will connect to your positive electrode (the anode) and the other will connect to your negative electrode (the cathode). Tape one end of each clip to the inside bottom of the tub. Make sure to leave enough space so that you can still open and close the clip.Advertisement
-
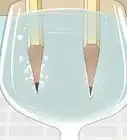
3Connect the electrodes. You will need 2 electrodes made from an non-reactive material that conducts electricity well. An optimum choice is platinum, but if you don’t have platinum electrodes lying around, you can use graphite. Shave the wood off of 2 pencils to make 2 graphite rods. Clip one to each alligator clip inside the tub.
- Graphite rods make great electrodes since they won’t dissolve in water and will conduct electricity.
Electrolyzing Water and Vinegar
-
1Mix a vinegar solution. Add 5 tablespoons (74 ml) of vinegar to 1⁄2 gallon (1.9 L) of water. The resulting solution will be slightly acidic and able to conduct a small amount of electricity. Pour the solution into the tub.[1]
- You may or may not use all of the solution. Just fill the tub most of the way.
-
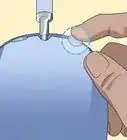
2Invert a test tube over each electrode. First, fill 2 test tubes with water. You can fill them from the tap, or with the water in your tub. Invert each test tube over one of the graphite electrodes and into the water. Be sure not to get any air bubbles in the test tubes. These tubes are meant to collect any hydrogen or oxygen that is formed.
- You will need to use a clamp to hold the test tubes in place over the rods.
-
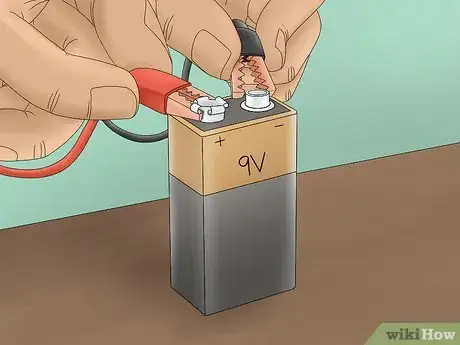
3Connect the alligator clips to a power supply. Connect the alligator clips (the side opposite the electrodes) to either terminal of a 9V battery. This will complete the circuit and allow electricity to flow. However, the current will be limited by the inability of the vinegar solution to conduct well. This will confirm that the acetic acid in vinegar does not promote electrolysis of water.[2]
- This limitation on current prohibits the water from being split into hydrogen and oxygen.
- Vinegar is a weak acid, so it doesn’t fully dissociate when dissolved in water, meaning there are fewer ions to conduct electricity.
Electrolyzing Water and Baking Soda
-
1Fill the tub with a sodium bicarbonate solution. Add 1 tbsp (21 g) of baking soda (sodium bicarbonate) to 1⁄2 gallon (1.9 L) of water. The baking soda will dissolve into electrolytes in the solution. These electrolytes will increase the amount of current that the water can conduct.
- Baking soda forms sodium and bicarbonate ions when dissociated.
-
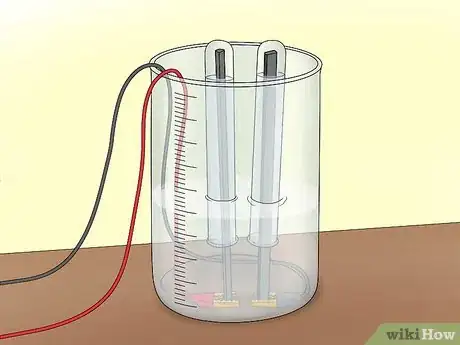
2Cover the electrodes. Fill 2 test tubes up with water from the tap or from the tub. Flip each test tube upside down to cover each electrode. Be sure not to allow any air into the test tubes. The tubes will be collecting the gases that you produce during electrolysis.
- Use a clamp to hold the test tubes in place over the electrodes.
-
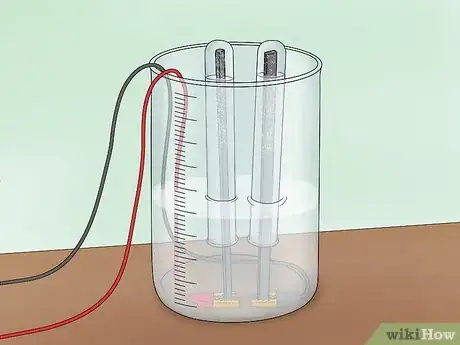
3Connect the power source. Connect 1 alligator clip to the positive terminal of a 9V battery. Connect the other to the negative terminal. This will create a circuit that allows current to flow through the electrolyte solution.
-
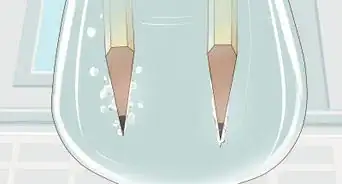
4Watch bubbles collect at the anode and cathode. As the current flows through the solution, it will split some of the water molecules. The result will be hydrogen gas (H2) and oxygen gas (O2). The oxygen gas, which has a charge of -2, will be attracted to a positive charge and will collect on the anode (an electron acceptor that attracts negative charges, called anions). The hydrogen gas, which has a charge of +1, will be attracted to a negative charge and will collect at the cathode (an electron acceptor that attracts positive charges, called cations).
- As the gases collect, they will bubble out of the water around their respective electrodes and be captured in the test tubes.
- You will notice that water is being displaced at the top of the test tube and replaced by the gases.
- Take note that the tube covering the cathode displaces twice as much water as the tube covering the anode. This is because there are twice as many hydrogen molecules being formed as oxygen molecules.
Warnings
- Hydrogen gas is highly flammable. In these small amounts, it is safe, but be cautious.⧼thumbs_response⧽
- Do not stick your hands in the water once the power source is connected.⧼thumbs_response⧽