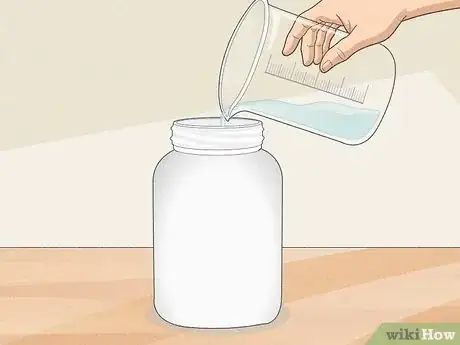This article was co-authored by wikiHow staff writer, Christopher M. Osborne, PhD. Christopher Osborne has been a wikiHow Content Creator since 2015. He is also a historian who holds a PhD from The University of Notre Dame and has taught at universities in and around Pittsburgh, PA. His scholarly publications and presentations focus on his research interests in early American history, but Chris also enjoys the challenges and rewards of writing wikiHow articles on a wide range of subjects.
There are 7 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 7,141 times.
Learn more...
The basic laboratory sea water formula, which is great for general lab use, requires only a 35 parts per thousand (ppt) mixture of household salt and tap water. More complex sea water formulas also exist, and require the use of more ingredients and distilled water, but are mixed in much the same manner. You can use any type of lab-made sea water to do a quick but fun density experiment with eggs—some will float, and some won’t!
Ingredients
- 35 g (1.2 oz) of kosher salt or table salt
- 965 g (34.0 oz) of tap water
- 26.518 g (0.9354 oz) of sodium chloride (NaCl)
- 2.447 g (0.0863 oz) of magnesium chloride (MgCl2)
- 3.305 g (0.1166 oz) of magnesium sulfate (MgSO4)
- 1.141 g (0.0402 oz) of calcium chloride (CaCl2)
- 0.725 g (0.0256 oz) of potassium chloride (KCl)
- 0.202 g (0.0071 oz) of sodium bicarbonate (NaHCO3)
- 0.083 g (0.0029 oz) of sodium bromide (NaBr)
- 965.579 g (34.0598 oz) of distilled water
Steps
Making Basic Sea Water
-
1Place a 1+ liter jar on a digital scale and press “tare.” Using the scale’s tare feature removes the mass of the jar from future measurements. This means the scale will only measure the mass of the materials you place inside the jar.[1]
- The container needs to be able to hold at least 1 L (34 fl oz) of water. A glass or clear plastic container is ideal, so you can see what’s going on inside, but isn’t absolutely necessary when you have a digital scale.
- You can still make sea water without a digital scale, but the results will be less precise. For your container, use a large measuring cup or beaker that is labeled to hold at least 1 L (34 fl oz) of liquid.
-
2Add kosher salt to the jar until the scale reads 35 g (1.2 oz). Kosher salt is the best choice here, as it produces a less cloudy sea water mixture. However, normal table salt is also acceptable.[2]
- If you don’t have a scale, add slightly under 6 tsp or 2 tbsp of kosher salt, or slightly over 6 tsp or 2 tbsp of table salt, to the jar.[3]
Advertisement -
3Pour in tap water until the scale reads 1,000 g (35 oz). Tap water is preferable to distilled or purified water, because it contains trace amounts of minerals like magnesium and calcium that are also found in true sea water. Use lukewarm water so the salt will dissolve more readily.[4]
- If you don’t have a scale, add tap water to your measuring cup or beaker (with the salt already in it) until you reach the 1 L (34 fl oz) mark. The mass of 1 L (34 fl oz) of water is 1,000 g (35 oz) or 1 kg.
-
4Stir the mixture until the salt is completely dissolved. This can take several minutes of stirring, so be patient! Once the salt is fully dissolved, the water will contain 35 parts per thousand (ppt) of salt—the basic laboratory approximation of sea water.[5]
- Think of it this way—in your 1,000 g (35 oz) mixture, 35 g (1.2 oz) come from the salt you added.
- Sea water salinity varies due to many factors, but 35 ppt is a good general approximation of typical salinity.
Mixing More Complex Sea Water
-
1Measure the non-water ingredients individually and add them to a beaker. Use your digital scale’s tare function to zero-out the mass of the vessel for each ingredient. Once you measure the needed amount of each ingredient, place it in a beaker that can hold at least 1 L (34 fl oz) of water.
- There are numerous lab-grade sea water recipes to choose from, depending on your specific needs.[6]
One, for example, uses the following to make 1,000 g (35 oz) of sea water:[7]
- 26.518 g of sodium chloride (NaCl)
- 2.447 g of magnesium chloride (MgCl2)
- 3.305 g of magnesium sulfate (MgSO4)
- 1.141 g of calcium chloride (CaCl2)
- 0.725 g of potassium chloride (KCl)
- 0.202 g of sodium bicarbonate (NaHCO3)
- 0.083 g of sodium bromide (NaBr)
- There are numerous lab-grade sea water recipes to choose from, depending on your specific needs.[6]
One, for example, uses the following to make 1,000 g (35 oz) of sea water:[7]
-
2Add distilled water to the beaker until the scale reads 1,000 g (35 oz). Put the beaker with the non-water ingredients in it on the digital scale and hit the “tare” button. Slowly pour in room-temperature distilled water until the scale’s mass reading reaches the desired amount—in this case, 1,000 g (35 oz) or 1 kg.[8]
- Because you’re being very particular about the specific ingredients of this sea water, it’s important to use distilled water that is completely free of impurities. This isn’t the case when making “simple” sea water, which uses plain tap water in order to introduce trace amounts of various minerals often found in sea water.
-
3Combine the ingredients completely by stirring them. Use a stirring stick or long spoon to dissolve the solids into the distilled water. It may take a few minutes of stirring to complete the process.[9]
- The solids will dissolve in lukewarm water faster than in cold water.
-
4Store the sea water for later use in a clean plastic container. If you’re not using the sea water for an experiment right away, pour it into a clean plastic container with a tight-fitting lid. Glass storage containers are not recommended because they may leach calcium into the sea water mixture.[10]
- Make sure there is no cleaning detergent residue inside the plastic container. If necessary, rinse it out several times with distilled water, then allow it to air dry.
Floating Eggs in Sea Water
-
1Mix a basic sea water solution (35 ppt) in a clear container. Place the container on a digital scale and press the tare button to zero-out the display. Add kosher or table salt until the scale reads 35 g (1.2 oz). Pour in lukewarm tap water until the scale reads 1,000 g (35 oz) or 1 kg. Stir the mixture until all the salt is dissolved.[11]
- Use a piece of tape to label this container as “sea water.”
- If you don’t have a digital scale, add slightly under 6 tsp (2 tbsp) of kosher salt or slightly over 6 tsp of table salt to a 1 L (34 fl oz) measuring cup, then add tap water until it reaches the 1 L (34 fl oz) line.
-
2Make 0 ppt, 20 ppt, 50 ppt, and 290 ppt salt water mixtures. Repeat the same process as before, putting each container on a digital scale, then adding kosher or table salt—add 0 g of salt to the first container, 20 g to the second, 50 g to the third, and 290 g to the fourth. Add tap water until the scale reads 1,000 g (35 oz). Stir each container thoroughly.[12]
- Label the 0 ppt solution “fresh water,” the 20 ppt as “brackish water,” the 50 ppt as “hypersaline sea water,” and the 290 ppt as “Dead Sea water.”
- If you don’t have a scale, 1 tsp of kosher salt equals roughly 6.0 g, and 1 tsp of table salt equals roughly 5.7 g.[13]
-
3Add a room-temperature egg to each labeled container. Choose eggs from the same batch that are roughly equal in size. Leave them on the counter for 1 hour if they have been refrigerated. Carefully lower them into the containers with a spoon so they don’t crack![14]
- Try not to splash any of the water out of the containers when adding the eggs.
-
4Compare the positions of the eggs in each container. You should see the following: the “fresh water” egg will sink to the bottom; the “brackish water” egg will sink or just barely float above the bottom; the “salt water” egg will float near the top; the “hypersaline salt water” egg will float at the top; and the “Dead Sea water” egg will float highest of all.[15]
- Why does this happen? Adding salt to water increases its density—that is, increases its mass in comparison to its volume. Once the density of the salt water is greater than the density of the egg, the egg floats!
- The formula for density is as follows: density (p) = mass (m) / volume (v).
Warnings
- Do not use these sea water mixtures in an aquarium! They do not have all the necessary minerals needed to sustain aquatic life. Buy a salt water aquarium mix and combine it with water according to the package instructions.⧼thumbs_response⧽
Things You’ll Need
Making Basic Sea Water
- Clear container
- Digital scale
- Spoon or stirring stick
Mixing More Complex Sea Water
- Clear beaker
- Digital scale
- Spoon or stirring stick
- Plastic storage container
Floating Eggs in Sea Water
- 5 clear containers
- Digital scale
- Large spoon
- 5 room-temperature eggs
References
- ↑ https://sciencing.com/make-sea-water-home-6368912.html
- ↑ https://manoa.hawaii.edu/exploringourfluidearth/physical/density-effects/density-temperature-and-salinity/practices-science-making-simulated-seawater
- ↑ https://www.traditionaloven.com/culinary-arts/cooking/multimenu.html
- ↑ https://sciencing.com/make-sea-water-home-6368912.html
- ↑ https://manoa.hawaii.edu/exploringourfluidearth/physical/density-effects/density-temperature-and-salinity/practices-science-making-simulated-seawater
- ↑ https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/artificial-seawater
- ↑ https://publishing.cdlib.org/ucpressebooks/view?docId=kt167nb66r&chunk.id=d3_5_ch06&toc.id=ch06&brand=eschol
- ↑ https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/artificial-seawater
- ↑ https://manoa.hawaii.edu/exploringourfluidearth/physical/density-effects/density-temperature-and-salinity/practices-science-making-simulated-seawater
- ↑ https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/artificial-seawater
- ↑ https://sciencing.com/make-sea-water-home-6368912.html
- ↑ https://www.weather.gov/jetstream/ll_eggs
- ↑ https://www.traditionaloven.com/culinary-arts/cooking/multimenu.html
- ↑ https://www.scientificamerican.com/article/salty-science-floating-eggs-in-water/
- ↑ https://www.weather.gov/jetstream/ll_eggs