This article was co-authored by wikiHow Staff. Our trained team of editors and researchers validate articles for accuracy and comprehensiveness. wikiHow's Content Management Team carefully monitors the work from our editorial staff to ensure that each article is backed by trusted research and meets our high quality standards.
There are 7 references cited in this article, which can be found at the bottom of the page.
The wikiHow Video Team also followed the article's instructions and verified that they work.
This article has been viewed 182,070 times.
Learn more...
Separating sand and salt is a fun science experiment you can do from home. If you were ever interested in the scientific idea of solubility, separating these two is a simple way of demonstrating the concept. Whether at home or in a classroom, it's an incredibly straightforward process, and you'll get a chance to see science in action.
Steps
Carrying Out the Experiment
-
1Gather your supplies. Because this is such a straightforward experiment, you won't need any lab gear or equipment. This is a cheap experiment. Here's a few things you'll need:
- Salt. Most households have table salt in the kitchen. If you're in a pinch, you can get salt packets from a fast food restaurant.
- Sand. Although it depends on where you live, sand should be very easy to find.
- A coffee filter and funnel. If the sand has a lot of chunks it, you should sift those out first using a strainer.[1]
- A pan and heating element. If you're in a chemistry lab, a flask and bunsen burner are arguably even better.[2] A second pan or plate is also recommended to catch the strained saltwater.
-
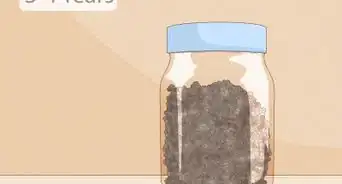
2Mix equal parts sand and salt into a pan. Measure out your portions carefully. Salt and sand mix together very well, and you can mix them together by shaking the pan around. If that doesn't work, stir it around until the two are thoroughly mixed.
- For the sake of keeping the experiment controlled, do your best to make the portions equal.
- You should have between 15g of salt and sand each.[3] This roughly equates to 1 tablespoon of each.[4]
- It's better to use smaller proportions. The experiment will prove the same point regardless, and it makes it easier to set up and clean up afterwards.
Advertisement -
3Add water to the sand and salt. If you have 10g each of sand and salt, add about 100mL of water, or just enough to cover the sand/salt mixture.
- Too much water will make the experiment take too long to boil off.
- Exact measurements aren't needed, but it can help keep the experiment consistent if you repeat it.
-
4Heat the mixture. Heat is the active ingredient when it comes to stirring up particles. A bit of heat will cause the salt to rile up and dissolve in the water. Stir it around if the salt you poured is in clumps. It may be interesting to watch the dissolution process occur, so keep your eyes peeled.
- Medium temperature on a stovetop will do nicely for this step.
- If you don't want to tamper with the dissolving process, you should leave the mixture untouched overnight.
- Make sure not to heat the water to the point of boiling! This will simply cause the water to evaporate, and you'll have to start from the beginning again.
-
5Strain the saltwater from the sand using a coffee filer and funnel. Set a funnel into a glass, making sure that it's big enough so that it rests on the rim. Next, set a coffee filter into the funnel. Pour the sand-water mixture into the coffee filter, then wait for it to drain through.[5]
- If you don't have any coffee filters, use a paper towel or a piece of cotton fabric, such as a handkerchief or bandana.
-
6Boil the saltwater. In order to separate the salt from the sand completely, you need to return the salt to its original state. This can be done by boiling the water. Put the pan on a stovetop and let the water boil. Wait until the water has boiled away completely. Turn off the heat. From there, you should be able to see the salt remaining in your pan.
- The boiling temperature of salt is much higher than water. For the sake of protecting your pot, you should keep the temperature relatively low on the stovetop. It may take longer to boil, but speed isn't worth the risk of damage.
- From here, you can retrieve the salt. Put the retrieved salt next to the sand for the sake of completion if you so desire.
Recording Your Observations
-
1Outline an experimental objective.[6] Objectives are often obvious, but it is good to have a concrete goal in mind when you conduct an experiment. In this case, you want to demonstrate the concept of solubility. The term "solubility" refers to something's ability to dissolve completely in a liquid.[7]
- Although a salt and sand experiment is generally pretty simple, you'll find you get more satisfaction by going through the paperwork.
-
2Make observations. An experiment is nothing without a critical eye. Making a habit of note taking during experiments will enrich the experience. You'll notice things you might otherwise overlook. Even obvious things should be written down. That way, you'll be able to make sense of it later. Observe the basic movements and changes in the experiment. Make note of the following.
- Although the salt dissolves in the heated water, the salt remains intact.
- The salt needs the water to be heated before it dissolves.
- The salt doesn't boil away with the water.
-
3Discuss the experiment.[8] By discussing an experiment in a group, you'll be able to compare your observations. If this experiment is happening in a class setting, it's possible that one of the experiments will turn out differently than the others. While this is likely a result of error, it's still interesting to see a new result and figure out where it came from.
- If you're by yourself, checking out a recording of the experiment on a streaming site like YouTube can be interesting. Even if you know the result, it is nonetheless worthwhile to see how someone else went about it.
-
4Reflect upon the experiment. As any successful scientist will tell you, most good science revolves around asking good questions. Look at your notes and think about the experience. What did you like about the experiment? Was there anything you might do differently if you had a second chance? Don't just think about the sand and salt, but all the things around it. What about different types of mixtures? A big part of good science is being inquisitive. Here are some questions you might ask:
- "Does the type of heating surface affect how well the salt dissolves?"
- "Would the experiment be different if I tried to dissolve it by stirring in room temperature water?"
- "Is the salt pure of water after boiling, or has the salt changed?"
-
5Expand upon the original experiment. Once you do the basic experiment, you should think of other questions you'd like to see answered. For example, how much longer does the process take if the portions are made unequal? Separating sand and salt is a very basic experiment, but the possibilities for a budding scientist are endless.
- For a lot of homebrewed experiments, baking soda is very fun to play around with. You could try adding that to your mixture next time.[9]
- Doing this as part of a group is more enjoyable than doing it on your own.
Community Q&A
-
QuestionHow does the salt water turn back to salt?
 Community AnswerThe water evaporates, leaving the salt behind.
Community AnswerThe water evaporates, leaving the salt behind. -
QuestionDoes the sand and salt mixture separate completely?
 Community AnswerNo, there may be some salt residue left even if it is washed with water several times.
Community AnswerNo, there may be some salt residue left even if it is washed with water several times. -
QuestionHow do you separate a mixture of salt and water?
 Community AnswerYou can separate water and salt using distillation. Boil your salt water so that the water evaporates and only the salt is left.
Community AnswerYou can separate water and salt using distillation. Boil your salt water so that the water evaporates and only the salt is left.
Warnings
- Although sand and salt aren't volatile chemicals, it will hurt if you let any of it get in your eyes. Protective eye gear is recommended if you happen to have any at your disposal.⧼thumbs_response⧽
References
- ↑ http://www.scientificamerican.com/article/bring-science-home-separate-solutions/
- ↑ http://www.rsc.org/learn-chemistry/resource/res00000386/separating-sand-and-salt?cmpid=CMP00005908
- ↑ http://www.chemheritage.org/percy-julian/teachers/7a4.html
- ↑ http://www.exploratorium.edu/cooking/convert/measurements.html
- ↑ http://www.scientificamerican.com/article/bring-science-home-separate-solutions/
- ↑ http://www.sciencebuddies.org/science-fair-projects/project_ideas/Chem_p016.shtml
- ↑ http://www.scientificamerican.com/article/bring-science-home-separate-solutions/
- ↑ http://www.chemheritage.org/percy-julian/teachers/7a4.html
- ↑ http://www.sciencebuddies.org/science-fair-projects/project_ideas/Chem_p016.shtml#makeityourown
About This Article
To separate sand and salt, start by pouring the sand and salt mixture into a pan. Then, add just enough water to cover the mixture. Heat the mixture over medium heat on a stovetop, which will cause the salt to dissolve in the water. Once the salt has completely dissolved, pour the mixture through a strainer to separate the sand and salt water. Finally, boil the salt water until all of the water evaporates and you're just left with the salt you started with. If you want to learn how to get the salt out of your pan when you're finished, keep reading the article!