This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
This article has been viewed 108,075 times.
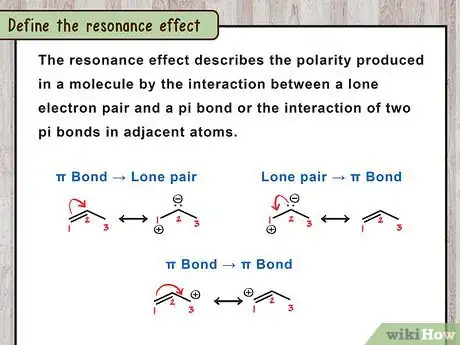
The resonance effect describes the polarity produced in a molecule by the interaction between a lone electron pair and a pi bond or the interaction of two pi bonds in adjacent atoms. In other words, resonance means that a molecule must be drawn with multiple Lewis structures, but actually exists in a hybrid state between the various configurations. It is usually found in molecules with conjugated double bonds or in molecules having at least one lone pair and one double bond.[1] Understanding resonance is important in understanding stability of the compound and its energy state.[2]
Steps
Understanding Resonance
-
1Define the resonance effect. The resonance effect is a chemical phenomenon observed in compounds characteristic of double bonds of organic compounds. Organic compounds that contain double bonds in their structure are usually made of the overlap of p-orbitals on two adjacent carbon atoms (referred to as pi bonds).[3]
- A single bond is often called a sigma bond and is present in compounds which have only one bond between the adjacent carbon atoms. Sigma bonds are usually lower in energy than pi bonds and also have higher symmetry than pi bonds.
-
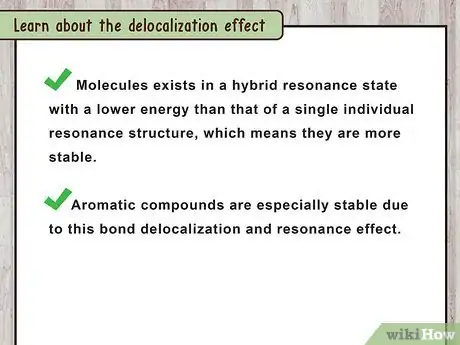
2Learn about the delocalization effect. The delocalization effect has been experimentally determined by measuring the heat of formation of the double bond containing compound alone and comparing it with the heat of formation of the sum of all the double bonds in the compound individually. The result of these measurements shows that the heat of formation of the whole molecule is lower than that of the sum of the heat of formation of its constituent double bonds measured singly.[4]
- This indicates that the molecules exists in a hybrid resonance state with a lower energy than that of a single individual resonance structure. In other words, they are more stable.
- Aromatic compounds are especially stable due to this bond delocalization and resonance effect.[5]
Advertisement -
3State the principles of resonance. Not all resonant structures are equally significant to the compound. There are a few principles involved that can help you determine how significant a resonance structure is.[6] ,[7]
- The rules of least charges: The resonant form with the lowest overall charge is the most significant.
- The octet principle: The resonance forms with a full octet are more significant than those lacking a full outer shell.
- Stabilization of positive charges: Forms where positive charges are acting on the least electronegative atom are the most significant.
- Stabilization of negative charges: Forms where negative charges are acting on the most electronegative atom are the most significant.
- Covalent bonds: The most significant resonance structure has the most covalent bonds.
Drawing Resonance Structures
-
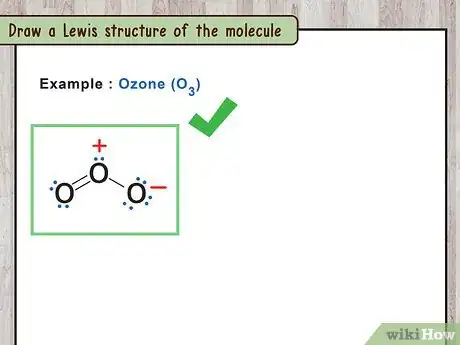
1Draw a Lewis structure of the molecule. A Lewis structure is a simplified representation of a molecule. It shows how atoms are bound together and their valence electron states.[8]
- Start by writing the chemical symbol of each element.
- Single bonds are represented with a line connecting the two atoms bound.
- Double bonds are represented by two lines and triple bonds by three.
- Valence electrons (electrons in the outer shell of the atom[9] ) are represented by dots next to the atom.
- Remember to indicate the overall charge of the molecule with a “+” or “-“ at the top right of the structure.
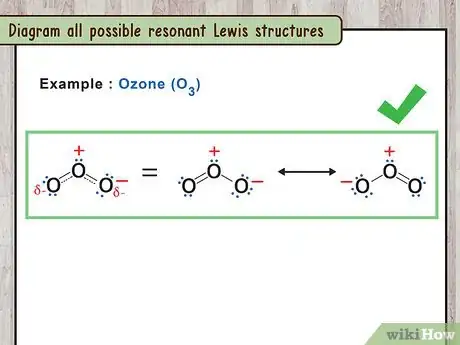
- For example: O3 has three oxygens all bound together. The oxygen in the middle is bound to the other two oxygens by one single bond and one double bond.
-
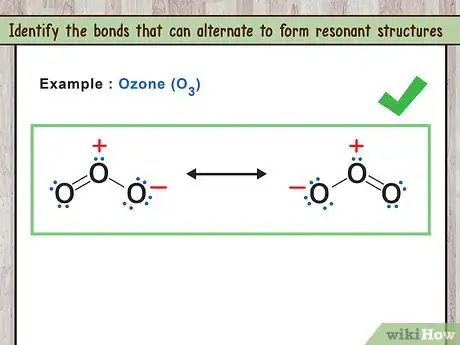
2Identify the bonds that can alternate to form resonant structures. Molecules that have resonant structures actually exist in a hybrid state between the different structures formed by the variations in bonds. While you may draw the various Lewis structures as separate molecules, that is just a way to pictorially represent them. Electrons that form double bonds can switch between atoms, slightly changing the way the structure is drawn.
- Bonds of this nature are said to be “delocalized”, as they are distributed evenly across all the atoms in the compound.[10]
- For example: O3 has two resonant structures. The double bond can be between the first and second oxygen or between the second and third oxygen.
-
3Diagram all possible resonant Lewis structures. Once you have identified the bonds that can alternate in the compound, you can draw the various Lewis structures for each version. It is also possible to draw a representative hybrid structure by using a dashed line where the bond could be either a single bond or a double bond.
- For example: You can draw two O3 structures with the two possible bond configurations or one O3 structure with dashed lines representing the bonds.[11]
- Draw a double-sided arrow between each structure to indicate that they are resonance structures.
Expert Q&A
-
QuestionWhat is the Mesomeric effect?
 Bess Ruff, MABess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Bess Ruff, MABess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Environmental Scientist The Mesomeric Effect is the effect of resonance on the acidity or basicity of a molecule or compound
The Mesomeric Effect is the effect of resonance on the acidity or basicity of a molecule or compound -
QuestionHow do I show the +R effect in a benzene structure?
 mukesh kumarCommunity AnswerWhen EDG (electron-donating groups) are directly attached to benzene ring, electron density on ortho and para position is increased. This phenomenon is known as +R effect, or +M vice versa. For example, in chlorobenzene the lone pair of chlorine atom delocalized with pi bond (pi electron) of the benzene ring; this effect is called ortho or para directive effect.
mukesh kumarCommunity AnswerWhen EDG (electron-donating groups) are directly attached to benzene ring, electron density on ortho and para position is increased. This phenomenon is known as +R effect, or +M vice versa. For example, in chlorobenzene the lone pair of chlorine atom delocalized with pi bond (pi electron) of the benzene ring; this effect is called ortho or para directive effect. -
QuestionWhat is the structure of resonance?
 Community AnswerResonance structures depends on the molecule. One way to know whether the structure is a resonance structure is to find out the bond length. If the bond length of the molecule is different from the usual bond lengths of e.g. double and single bonds, the structure should be a resonance structure.
Community AnswerResonance structures depends on the molecule. One way to know whether the structure is a resonance structure is to find out the bond length. If the bond length of the molecule is different from the usual bond lengths of e.g. double and single bonds, the structure should be a resonance structure.
References
- ↑ http://www.masterorganicchemistry.com/2011/12/22/in-summary-resonance/
- ↑ https://brilliant.org/wiki/inductive-effect-electromeric-efffect-resonance/#resonance
- ↑ http://goldbook.iupac.org/M03844.html
- ↑ http://www.masterorganicchemistry.com/2011/12/22/in-summary-resonance/
- ↑ http://crab.rutgers.edu/~alroche/Ch16.pdf
- ↑ http://www.masterorganicchemistry.com/2011/12/22/in-summary-resonance/
- ↑ https://brilliant.org/wiki/inductive-effect-electromeric-efffect-resonance/#resonance
- ↑ http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/Lewis_Theory_of_Bonding/Lewis_Structures
- ↑ http://www.chem.sc.edu/faculty/shimizu/333/Chem_333/1a.i.html