This article was co-authored by Chris Hasegawa, PhD. Dr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
There are 8 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 14,267 times.
The pH level measures how acidic or alkaline a material is. Distilled water is neutral and measures 7.0 on the pH scale. Materials from 1-6 are acidic and materials from 8-14 are alkaline. If you'd like to find out for yourself where a material falls on the pH scale, there are several ways you can test for pH using store-bought test strips or a home concoction of red cabbage extract.
Steps
Using Litmus Test Strips for Liquids
-
1Buy a box of pH test strips. These are available on the internet or at pharmacies. Pool supply stores should also carry this equipment.[1]
- Look on the box to make sure these test strips cover the whole pH range. Some test strips only cover a portion of the pH scale.
- You need the color code on the box to accurately measure pH, so save the packaging after you open the box.
-
2Read the instructions on the test strip box. All pH strips work similarly, but might have slightly different instructions. For example, some need to be held in the liquid for several seconds to get an accurate reading, and some produce a reading instantly. [2]Advertisement
-
3Pour the liquid you want to test into a cup. You only need a small amount to test a liquid’s pH, so no need to fill the whole cup. Pour just enough to cover the bottom of the cup.[3]
- Rinse the cup with distilled water before filling it to make sure there are no contaminants that will throw off your results.
- Wear gloves and goggles if you don’t know what this liquid is. Acidic or alkaline liquids can burn your skin or eyes and require medical attention.
-
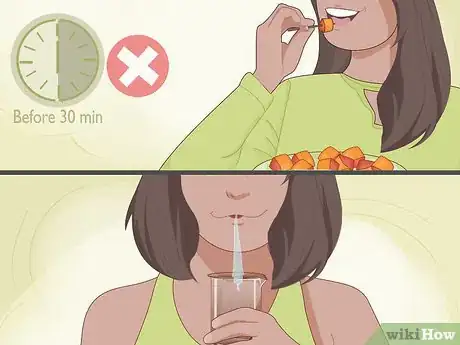
4Spit into a spoon if you're testing your body pH. You can also use test strips to measure your body pH level. Don't put the strips in your mouth because this can spread bacteria into your body. Instead, spit into a spoon and follow the directions for dipping in a test strip.[4]
- Don't eat, drink, or brush your teeth for 30 minutes before doing this. These activities can affect your mouth pH.
-
5Dip the test strip into the liquid. Only insert the tip into the liquid. Leave the strip in the liquid for as long as the box instructs. Within a few seconds, you’ll notice that the test strip is reacting to the liquid and changing color.[5]
- When you pull the strip out, hold it above the cup for a few seconds so any excess liquid drips back in. Also wipe any remaining liquid on the inside of the cup.
- If the test strip doesn’t react at all, you may have a faulty batch. Try several and if you get no reaction, return them to the store.
-
6Match the color of the reaction to the color code on the box. When the test strip reacts with the liquid, the new color pattern indicates the pH of the liquid. The color pattern will correspond with one of the color patterns on the box. Each pH level from 0 to 14 has a unique pattern. Hold your test strip up to the box and see which color pattern has appeared on the strip. The corresponding number indicates what pH this liquid is.[6] [7]
- If you’ve lost the test strip packaging, there are universal color codes available online. Search for one on the internet to conduct the test.
Measuring the pH of a solid
-
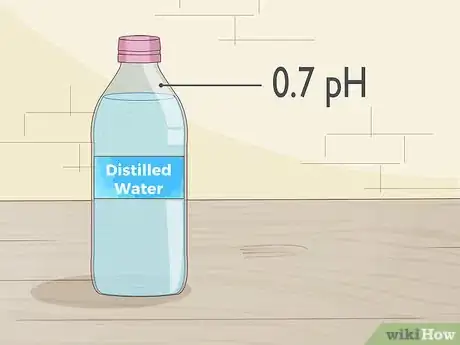
1Get a container of distilled water. Distilled water is purified, so you know its pH is exactly 7.0. Only use distilled water for this step. Water from the tap is usually slightly acidic, so it will make anything you measure more acidic and prevent you from getting an accurate pH measurement.[8]
- Most pharmacies carry containers of distilled water. There are also water companies that will deliver containers of purified water to your home.
-
2Blend any food items you're testing into a paste. Soil or powder will easily mix into water, but food items need to be broken down for an accurate pH reading. Scoop the food into a blender or processor and blend until it’s reduced to a paste. Add distilled water if the item is dry or doesn’t reduce into a paste consistency.[9]
- Be careful when scooping food out of the processor. Avoid the blades so you don’t get cut.
-
3Mix the sample into a small amount of distilled water. The distilled water should only be a maximum of 25% of the sample volume so it doesn't dilute the pH. So if your sample is 100 millilitres (3.5 imp fl oz; 3.4 fl oz), only add 25 millilitres (0.88 imp fl oz; 0.85 fl oz) of water. Use a measuring cup to check the volume. Mix the sample together.[10]
- If you’re testing a food item that came in liquid, also mix in some of that liquid. This gives you a comprehensive reading of the pH of the item.
-
4Dip a pH test strip into the mixture. You don’t have to submerge the entire test strip. Just inserting the tip will measure the pH. Leave the strip in the liquid for as long as the box instructs. Notice how the color begins changing.[11]
- When you remove the test strip, leave it over the cup for a few seconds to let any excess liquid drip off.
-
5Match the color reaction to the color code on the box. In a few seconds, the test strip will react with the liquid and change color. Each pH level from 0 to 14 has a unique pattern. The color pattern corresponds with one of the color patterns printed on the box. Hold the test strip up to the box and see which color pattern matches. The corresponding number is the pH of the material.[12] [13]
- If you’ve lost the test strip packaging, there are universal color codes available online. Search for one on the internet to conduct the test.
Testing Liquids with Red Cabbage Juice
-
1Buy a container of red cabbage extract. Search the internet for red cabbage extract sellers. This liquid has a unique characteristic because it reacts and changes color according to the pH of a liquid it’s mixed with. This won’t give you an exact measurement, but it will give you a general idea of the pH for liquids.[14]
- Using red cabbage as a pH indicator is a popular classroom experiment, so school supply stores may also stock this.
-
2Fill a cup with the liquid you’re testing. Measure 100 millilitres (3.5 imp fl oz; 3.4 fl oz) of the liquid you’re testing and pour it into a cup. Using a clear glass cup or beaker works best so you can get a clear view of the color change.[15]
- Wear goggles during this step to protect your eyes from any splashes.
-
3Add 50 millilitres (1.8 imp fl oz; 1.7 fl oz) of red cabbage extract. Wait a few seconds for the cabbage juice to react with the liquid. Stir the mixture lightly to help the reaction along.[16]
-
4Assess the color of the mixture. After a few seconds, the cabbage juice should change color according to the pH of the liquid you’ve tested. Use the corresponding guide to assess the pH of this liquid.[17]
- If the mixture is pink, the pH is 1-2.
- If the mixture is dark red, the pH is 3-4.
- If the mixture is violet, the pH is 5-7.
- If the mixture is blue, the pH is 8.
- If the mixture is blue-green, the pH is 9-10.
- If the mixture is green-yellow, the pH is 11-12.
-
5Use fresh cabbage juice for each liquid you test. The cabbage juice is contaminated after one use and won’t give you an accurate reading again. Pour out the liquid and rinse the cup with water. Alternatively, you could use a fresh cup.[18]
- If you’re testing several liquids, lay out several empty cups and fill each one with a different liquid. Label each cup so you remember which liquid is in each. Then pour the cabbage juice into each cup one at a time to test several at once.
Expert Q&A
-
QuestionHow do pH meters work?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean pH meters get placed directly in the solution or item that you'd like to test. Then, the meter tells you what the ion concentration is.
pH meters get placed directly in the solution or item that you'd like to test. Then, the meter tells you what the ion concentration is. -
QuestionWhat is the difference between indicator and litmus paper?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean Both litmus and indicator paper use different color-coded systems to measure pH. Litmus paper measures pH by turning pink or blue, while indicator strips follow a special color-coded chart that's included on the box.
Both litmus and indicator paper use different color-coded systems to measure pH. Litmus paper measures pH by turning pink or blue, while indicator strips follow a special color-coded chart that's included on the box.
Things You'll Need
Using Litmus Test Strips for Liquids
- Litmus pH test strips
- Cup
- Distilled water
- Gloves
- Goggles
Measuring the pH of a solid
- Litmus pH test strips
- Distilled water
- Cup
- Measuring cup
Testing Liquids with Red Cabbage Juice
- Red cabbage extract
- Clear cup or glass
- Measuring cup
- Goggles
Warnings
- Always wear gloves and goggles when working with chemicals. Acidic or alkaline materials can injure your skin and eyes.⧼thumbs_response⧽
- If you get any chemicals on your skin, flush the area with running water for several minutes.⧼thumbs_response⧽
References
- ↑ https://sciencing.com/use-ph-strips-test-water-7869805.html
- ↑ https://sciencing.com/use-ph-strips-test-water-7869805.html
- ↑ https://sciencing.com/use-ph-strips-test-water-7869805.html
- ↑ https://sciencing.com/methods-testing-ph-liquids-5809509.html
- ↑ https://sciencing.com/use-ph-strips-test-water-7869805.html
- ↑ https://youtu.be/JpQw-_1jLzs?t=36
- ↑ Chris Hasegawa, PhD. Retired Science Professor & Dean. Expert Interview. 29 July 2021.
- ↑ https://www.usgs.gov/special-topic/water-science-school/science/ph-and-water?qt-science_center_objects=0#qt-science_center_objects
- ↑ https://youtu.be/QMfCYkZPvXg?t=68
- ↑ https://assets.thermofisher.com/TFS-Assets/LSG/brochures/pH-Measurement-Handbook-S-PHREFBK-E.pdf
- ↑ https://sciencing.com/use-ph-strips-test-water-7869805.html
- ↑ https://youtu.be/JpQw-_1jLzs?t=36
- ↑ Chris Hasegawa, PhD. Retired Science Professor & Dean. Expert Interview. 29 July 2021.
- ↑ https://sciencing.com/methods-testing-ph-liquids-5809509.html
- ↑ https://web.stanford.edu/~ajspakow/downloads/outreach/ph-student-9-30-09.pdf
- ↑ https://web.stanford.edu/~ajspakow/downloads/outreach/ph-student-9-30-09.pdf
- ↑ https://web.stanford.edu/~ajspakow/downloads/outreach/ph-student-9-30-09.pdf
- ↑ https://web.stanford.edu/~ajspakow/downloads/outreach/ph-student-9-30-09.pdf