2-Methyl-1-butanol
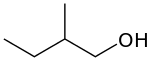
2-Methyl-1-butanol (IUPAC name, also called active amyl alcohol) is an organic compound with the formula CH3CH2CH(CH3)CH2OH. It is one of several isomers of amyl alcohol. A colorless liquid, it occurs naturally in trace amounts and has attracted some attention as a potential biofuel, exploiting its hydrophobic (gasoline-like) and branched structure. It is chiral.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylbutan-1-ol | |
| Other names
2-Methyl-1-butanol Active amyl alcohol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.809 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12O | |
| Molar mass | 88.148 g/mol |
| Appearance | colorless liquid |
| Density | 0.8152 g/cm3 |
| Melting point | −117.2 °C (−179.0 °F; 156.0 K) |
| Boiling point | 127.5 °C (261.5 °F; 400.6 K) |
| 31 g/L | |
| Solubility | organic solvents |
| Vapor pressure | 3 mm Hg |
| Viscosity | 4.453 mPa·s |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-356.6 kJ·mol−1 (liquid) -301.4 kJ·mol−1 (gas) |
| Hazards | |
| 385 °C (725 °F; 658 K) | |
| Related compounds | |
Related compounds |
Amyl alcohol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Occurrence
2-Methyl-1-butanol is a component of many mixtures of commercial amyl alcohols. It is one of the many components of the aroma of various fungi and fruit, e.g., the summer truffle, tomato,[4] and cantaloupe.[5][6]
Production and reactions
2-Methyl-1-butanol has been produced from glucose by genetically modified E. coli. 2-Keto-3-methylvalerate, a precursor to threonine, is converted to the target alcohol by the sequential action of 2-keto acid decarboxylase and dehydrogenase.[7] It can be derived from fusel oil (because it occurs naturally in fruits such as grapes[8]) or manufactured by either the oxo process or via the halogenation of pentane.[2]
See also
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3–374, 5–42, 6–188, 8–102, 16–22, ISBN 0-8493-0594-2
- McKetta, John J.; Cunningham, William Aaron (1977), Encyclopedia of Chemical Processing and Design, vol. 3, Boca Raton, Florida: CRC Press, pp. 279–280, ISBN 978-0-8247-2480-1, retrieved 2009-12-14
- Xiong, Ren-Gen; You, Xiao-Zeng; Abrahams, Brendan F.; Xue, Ziling; Che, Chi-Ming (2001). "Enantioseparation of Racemic Organic Molecules by a Zeolite Analogue". Angewandte Chemie International Edition. 40 (23): 4422–4425. doi:10.1002/1521-3773(20011203)40:23<4422::AID-ANIE4422>3.0.CO;2-G. PMID 12404434.
- Buttery, Ron G.; Teranishi, Roy; Ling, Louisa C. (1987). "Fresh tomato aroma volatiles: A quantitative study". Journal of Agricultural and Food Chemistry. 35 (4): 540–544. doi:10.1021/jf00076a025.
- Dı́Az, P.; Ibáñez, E.; Señoráns, F.J; Reglero, G. (2003). "Truffle Aroma Characterization by Headspace solid-phase microextraction". Journal of Chromatography A. 1017 (1–2): 207–214. doi:10.1016/j.chroma.2003.08.016. PMID 14584705.
- Beaulieu, John C.; Grimm, Casey C. (2001). "Identification of Volatile Compounds in Cantaloupe at Various Developmental Stages Using Solid Phase Microextraction". Journal of Agricultural and Food Chemistry. 49 (3): 1345–1352. doi:10.1021/jf0005768. PMID 11312862.
- Atsumi, Shota; Hanai, Taizo; Liao, James C. (2008). "Non-Fermentative Pathways for Synthesis of Branched-Chain Higher Alcohols as Biofuels". Nature. 451 (7174): 86–89. Bibcode:2008Natur.451...86A. doi:10.1038/nature06450. PMID 18172501. S2CID 4413113.
- Howard, Philip H. (1993), Handbook of Environmental Fate and Exposure Data for Organic Chemicals, vol. 4, Boca Raton, Florida: CRC Press, pp. 392–396, ISBN 978-0-87371-413-6, retrieved 2009-12-14