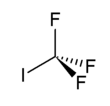
Trifluoroiodomethane
Trifluoroiodomethane, also referred to as trifluoromethyl iodide is a halomethane with the formula CF3I. It is an experimental alternative to Halon 1301 (CBrF3) in unoccupied areas.[1] It would be used as a gaseous fire suppression flooding agent for in-flight aircraft and electronic equipment fires.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trifluoro(iodo)methane | |||
| Other names
Trifluoroiodomethane Iodotrifluoromethane Monoiodotrifluoromethane Trifluoromethyl iodide Perfluoromethyl iodide Freon 13T1 | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.017.286 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CF3I | |||
| Molar mass | 195.91 g/mol | ||
| Appearance | Colorless odorless gas | ||
| Density | 2.5485 g/cm3 at -78.5 °C 2.3608 g/cm3 at -32.5 °C | ||
| Melting point | −110 °C (−166 °F; 163 K) | ||
| Boiling point | −22.5 °C (−8.5 °F; 250.7 K) | ||
| Slightly | |||
| Vapor pressure | 541 kPa | ||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Warning | |||
| H341 | |||
| P201, P202, P281, P308+P313, P405, P501 | |||
| Supplementary data page | |||
| Trifluoroiodomethane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Chemistry
It is used in the rhodium-catalyzed α-trifluoromethylation of α,β-unsaturated ketones.[2]
It can be used as a new generation fire extinguishing agent to replace Halon in fire protection systems.[3] The mechanism of extinguishing fires for CF3I is active and primarily based on interruption of the chain reaction in the combustion area of the flame by so-called "negative" catalytic action.[4] It is also used as an eco-friendly insulation gas to replace SF6 in electrical power industry.[5]
In the presence of sunlight or at temperatures above 100 °C it can react with water, forming hazardous by-products such as hydrogen fluoride (HF), hydrogen iodide (HI) and carbonyl fluoride (COF2).
Environmental effects
Trifluoroiodomethane contains carbon, fluorine, and iodine atoms. Although iodine is several hundred times more efficient at destroying stratospheric ozone than chlorine, experiments have shown that because the weak C-I bond breaks easily under the influence of water (owing to the electron-attracting fluorine atoms), trifluoroiodomethane has an ozone depleting potential less than one-thousandth that of Halon 1301 (0.008-0.01). Its atmospheric lifetime, at less than 1 month, is less than 1 percent that of Halon 1301, and less even than hydrogen chloride formed from volcanoes.
There is, however, still the problem of the C-F bonds absorbing in the atmospheric window.[6] However, the IPCC has calculated the 100-year global warming potential of trifluoroiodomethane to be 0.4 (i.e., 40% of that of CO2).[7]
References
- Vitali, Juan. "Halon Substitute Protects Aircrews and the Ozone Layer". www.afrlhorizons.com. Archived from the original on 11 July 2007. Retrieved 2017-09-06.
- "Trifluoroiodomethane 171441". Sigma-Aldrich. Retrieved 2017-09-06.
- "Fire extinguishing agents trifluoroiodomethane/CF3I". beijingyuji. Retrieved 2018-09-20.
- "CFI rim seal fire protection for floating roof tanks" (PDF). 2018-09-20.
- Katagiri, H.; Kasuya, H.; Mizoguchi, H.; Yanabu, S. (October 2008). "Investigation of the Performance of CF3I Gas as a Possible Substitute for SF6". IEEE Transactions on Dielectrics and Electrical Insulation. 15 (5): 1424–1429. doi:10.1109/TDEI.2008.4656252. S2CID 21905295.
- Shimanouchi, T. (July 1977). "Tables of molecular vibrational frequencies. Consolidated volume II". Journal of Physical and Chemical Reference Data. 6 (3): 993–1102. Bibcode:1977JPCRD...6..993S. doi:10.1063/1.555560.
- Ramfjord, Birgit (2012-03-05). "Listing of GWP Values as per Report IPCC WG1 AR4" (PDF). Swedish Defence Materiel Administration. Archived from the original (PDF) on 13 March 2016. Retrieved 7 September 2017.
Further reading
- National Research Council (US) Subcommittee on Iodotrifluoromethane (2004). Iodotrifluoromethane. doi:10.17226/11090. ISBN 978-0-309-09307-1. PMID 25032315.
- Solomon, Susan; Burkholder, James B.; Ravishankara, A. R.; Garcia, Rolando R. (1994). "Ozone depletion and global warming potentials of CF3I". Journal of Geophysical Research. 99 (D10): 20929. doi:10.1029/94JD01833.
- Duan, Y. Y.; Shi, L.; Sun, L. Q.; Zhu, M. S.; Han, L. Z. (1 March 2000). "Thermodynamic Properties of Trifluoroiodomethane (CF3I)". International Journal of Thermophysics. 21 (2): 393–404. doi:10.1023/A:1006683529436. S2CID 118125837.
- Duan, Yuan-Yuan; Shi, Lin; Zhu, Ming-Shan; Han, Li-Zhong (January 1999). "Surface tension of trifluoroiodomethane (CF3I)". Fluid Phase Equilibria. 154 (1): 71–77. doi:10.1016/S0378-3812(98)00439-7.
- Duan, Y. Y.; Sun, L. Q.; Shi, L.; Zhu, M. S.; Han, L. Z. (1 September 1997). "Thermal Conductivity of Gaseous Trifluoroiodomethane (CF3I)". Journal of Chemical & Engineering Data. 42 (5): 890–893. doi:10.1021/je9700378.
- Duan, Yuan-Yuan; Shi, Lin; Zhu, Ming-Shan; Han, Li-Zhong (1 May 1999). "Critical Parameters and Saturated Density of Trifluoroiodomethane (CF3I)". Journal of Chemical & Engineering Data. 44 (3): 501–504. doi:10.1021/je980251b.
- Markgraf, Stewart J.; Wells, J. R.; Wiseman, Floyd L. (30 April 1996). Chamber Studies of Photolysis and Hydroxyl Radical Reactions of Trifluoroiodomethane. DTIC ADA318474.
External links
- National Research Council (US) Subcommittee on Iodotrifluoromethane (2004). "Physical and Chemical Properties And Efficacy". Iodotrifluoromethane. pp. 15–17. doi:10.17226/11090. ISBN 978-0-309-09307-1. PMID 25032315.
- Data sheet (in Japanese)
- Material Safety Data Sheet CF3I (in English)
- CF3I can be used as fire extinguishing agent