Carboxyamidotriazole
Carboxyamidotriazole is a calcium channel blocker that blocks voltage-gated and ligand-gated calcium channels and has been investigated as an anti-cancer drug in vitro.[1][2]
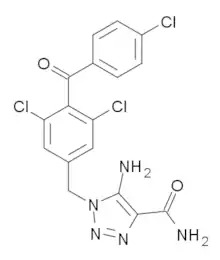 | |
| Names | |
|---|---|
| Preferred IUPAC name
5-Amino-1-{[3,5-dichloro-4-(4-chlorobenzoyl)phenyl]methyl}-1H-1,2,3-triazole-4-carboxamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.231.281 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H12Cl3N5O2 | |
| Molar mass | 424.66848 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Figg, W. D.; Cole, K. A.; Reed, E.; Steinberg, S. M.; Piscitelli, S. C.; Davis, P. A.; Soltis, M. J.; Jacob, J.; Boudoulas, S.; Goldspiel, B. (1995). "Pharmacokinetics of orally administered carboxyamido-triazole, an inhibitor of calcium-mediated signal transduction". Clinical Cancer Research. 1 (8): 797–803. PMID 9816048.
- Bonnefond, M. L.; Florent, R.; Lenoir, S.; Lambert, B.; Abeilard, E.; Giffard, F.; Louis, M. H.; Elie, N.; Briand, M.; Vivien, D.; Poulain, L.; Gauduchon, P.; n'Diaye, M. (2018). "Inhibition of store-operated channels by carboxyamidotriazole sensitizes ovarian carcinoma cells to anti-BCLXL strategies through MCL-1 down-regulation". Oncotarget. 9 (74): 33896–33911. doi:10.18632/oncotarget.26084. PMC 6188062. PMID 30338034.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.