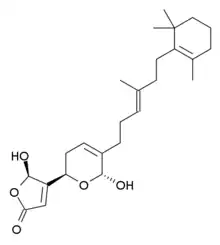
Manoalide
Manoalide is a calcium channel blocker. It has antibiotic, analgesic and anti-inflammatory effects and is found in some sponges, including the West Pacific species Luffariella variabilis.[1] Its functions are made possible by the permanent blockage of phospholipase A2 and C[2] with lysine residues. This could be made possible through the functional groups incorporated in gamma-hydroxybutenolide, alpha-hydroxydihydropyran and the trimethylcyclohexenyl. The gamma-hydroxybutenolide ring is present in the reaction between manoalide and phospholipase A2, the hemiacetal in alpha-hydroxydihydropyran is needed for permanent binding and hydrophobic trimethylcyclohexenyl ring makes it possible for non-bonded interactions to interact between manoalide and phospholipase A2 to strengthen the reaction. [3] Due to its potential of permanent inhibition, it was made possible for it to take part in oral cancer[4] and Hepatitis C[5] research.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(5R)-5-Hydroxy-4-{(2R,6R)-6-hydroxy-5-[(3E)-4-methyl-6-(2,6,6-trimethylcyclohex-1-en-1-yl)hex-3-en-1-yl]-3,6-dihydro-2H-pyran-2-yl}furan-2(5H)-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C25H36O5 | |
| Molar mass | 416.55034 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Brusca, Richard C. and Brusca, Gary J. Invertebrates. 2nd ed. Sinauer Associates, 2002. p. 202.
- Oxford dictionary of biochemistry and molecular biology. Cammack, Richard, Ph. D. (Rev. ed.). Oxford: Oxford University Press. 2006. ISBN 978-1-61344-113-8. OCLC 743217704.
{{cite book}}: CS1 maint: others (link) - "Molecular Pharmacology: 98 (6)". Molecular Pharmacology. 98 (6). 2020-12-01. ISSN 0026-895X.
- Wang, Hui-Ru; Tang, Jen-Yang; Wang, Yen-Yun; Farooqi, Ammad Ahmad; Yen, Ching-Yu; F. Yuan, Shyng-Shiou; Huang, Hurng-Wern; Chang, Hsueh-Wei (2019). "Manoalide Preferentially Provides Antiproliferation of Oral Cancer Cells by Oxidative Stress-Mediated Apoptosis and DNA Damage". Cancers. 11 (9): 1303. doi:10.3390/cancers11091303. PMC 6770486. PMID 31487907.
- Salam, Kazi Abdus; Furuta, Atsushi; Noda, Naohiro; Tsuneda, Satoshi; Sekiguchi, Yuji; Yamashita, Atsuya; Moriishi, Kohji; Nakakoshi, Masamichi; Tsubuki, Masayoshi; Tani, Hidenori; Tanaka, Junichi (2012). "Inhibition of Hepatitis C Virus NS3 Helicase by Manoalide". Journal of Natural Products. 75 (4): 650–4. doi:10.1021/np200883s. PMID 22394195.