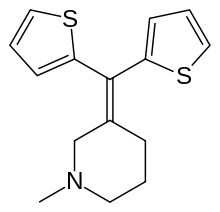
Tipepidine
Tipepidine (INN) (brand names Asverin, Antupex, Asvelik, Asvex, Bitiodin, Cofdenin A, Hustel, Nodal, Sotal), also known as tipepidine hibenzate (JAN), is a synthetic, non-opioid antitussive and expectorant of the thiambutene class.[1][2] It acts as an inhibitor of G protein-coupled inwardly-rectifying potassium channels (GIRKs).[3] The drug was discovered in the 1950s,[4] and was developed in Japan in 1959.[5] It is used as the hibenzate and citrate salts.[1][5]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H17NS2 |
| Molar mass | 275.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
The usual dose is 20 mg every 4–6 hours. Possible side effects of tipepidine, especially in overdose, may include drowsiness, vertigo, delirium, disorientation, loss of consciousness, and confusion.[5]
Tipepidine has been investigated as a potential psychiatric drug. It is being investigated in depression,[3][6][7] obsessive-compulsive disorder,[8] and attention-deficit hyperactivity disorder (ADHD).[9][10][11] Through inhibition of GIRK channels, tipepidine increases dopamine levels in the nucleus accumbens, but without increasing locomotor activity or producing methamphetamine-like behavioral sensitization, and this action appears to be at least partly responsible for its antidepressant-like effects in rodents.[12][13]
See also
References
- Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1988–. ISBN 978-0-412-46630-4.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1649–. ISBN 978-3-88763-075-1.
- Kawaura K, Ogata Y, Inoue M, Honda S, Soeda F, Shirasaki T, Takahama K (December 2009). "The centrally acting non-narcotic antitussive tipepidine produces antidepressant-like effect in the forced swimming test in rats". Behavioural Brain Research. 205 (1): 315–318. doi:10.1016/j.bbr.2009.07.004. PMID 19616036. S2CID 29236491.
- ES 272195, "Procedure for the preparation of a new piperidine derivative of anti-nutritional activity", published 1 March 1962, assigned to Antonio Gallardo SA.
- Imai Y, Ishii W, Endo A, Arakawa C, Kohira R, Fujita Y, et al. (October 2011). "Tipepidine hibenzate intoxication". Pediatrics International. 53 (5): 779–781. doi:10.1111/j.1442-200X.2010.03297.x. PMID 21955016. S2CID 205484528.
- Kawaura K, Honda S, Soeda F, Shirasaki T, Takahama K (May 2010). "[Novel antidepressant-like action of drugs possessing GIRK channel blocking action in rats]". Yakugaku Zasshi. 130 (5): 699–705. doi:10.1248/yakushi.130.699. PMID 20460867.
- Sasaki T, Hashimoto K, Tachibana M, Kurata T, Kimura H, Komatsu H, et al. (2014). "Tipepidine in adolescent patients with depression: a 4 week, open-label, preliminary study". Neuropsychiatric Disease and Treatment. 10: 719–722. doi:10.2147/NDT.S63075. PMC 4015794. PMID 24833905.
- Honda S, Kawaura K, Soeda F, Shirasaki T, Takahama K (January 2011). "The potent inhibitory effect of tipepidine on marble-burying behavior in mice". Behavioural Brain Research. 216 (1): 308–312. doi:10.1016/j.bbr.2010.08.010. PMID 20713091. S2CID 21118027.
- Sasaki T, Hashimoto K, Tachibana M, Kurata T, Okawada K, Ishikawa M, et al. (2014). "Tipepidine in children with attention deficit/hyperactivity disorder: a 4-week, open-label, preliminary study". Neuropsychiatric Disease and Treatment. 10: 147–151. doi:10.2147/NDT.S58480. PMC 3908907. PMID 24493927.
- Hashimoto K, Sasaki T (February 2015). "Old drug tipepidine as new hope for children with ADHD". The Australian and New Zealand Journal of Psychiatry. 49 (2): 181–182. doi:10.1177/0004867414553952. PMID 25280911. S2CID 8496750.
- Dehbozorghi S, Bagheri S, Moradi K, Shokraee K, Mohammadi MR, Akhondzadeh S (November 2019). "Efficacy and safety of tipepidine as adjunctive therapy in children with attention-deficit/hyperactivity disorder: Randomized, double-blind, placebo-controlled clinical trial". Psychiatry and Clinical Neurosciences. 73 (11): 690–696. doi:10.1111/pcn.12913. PMID 31294924. S2CID 195879810.
- Hamasaki R, Shirasaki T, Soeda F, Takahama K (November 2013). "Tipepidine activates VTA dopamine neuron via inhibiting dopamine D₂ receptor-mediated inward rectifying K⁺ current". Neuroscience. 252: 24–34. doi:10.1016/j.neuroscience.2013.07.044. PMID 23896570. S2CID 207253362.
- Hamao K, Kawaura K, Soeda F, Hamasaki R, Shirasaki T, Takahama K (May 2015). "Tipepidine increases dopamine level in the nucleus accumbens without methamphetamine-like behavioral sensitization". Behavioural Brain Research. 284: 118–124. doi:10.1016/j.bbr.2015.02.012. PMID 25687844. S2CID 22324207.