List of therapeutic monoclonal antibodies
Therapeutic, diagnostic and preventive monoclonal antibodies are clones of a single parent cell. When used as drugs, the International Nonproprietary Names (INNs) end in -mab. The remaining syllables of the INNs, as well as the column Source, are explained in Nomenclature of monoclonal antibodies.
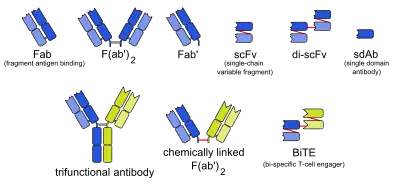
Types of monoclonal antibodies with other structures than naturally occurring antibodies.
The abbreviations in the column Type are as follows:
- mab: whole monoclonal antibody
- Fab: fragment, antigen-binding (one arm)
- F(ab')2: fragment, antigen-binding, including hinge region (both arms)
- Fab': fragment, antigen-binding, including hinge region (one arm)
- Variable fragments:
- scFv: single-chain variable fragment
- di-scFv: dimeric single-chain variable fragment
- sdAb: single-domain antibody
- BsAb: bispecific monoclonal antibody:
- 3funct: trifunctional antibody
- BiTE: bi-specific T-cell engager
This list of over 500 monoclonal antibodies includes approved and investigational drugs as well as drugs that have been withdrawn from market; consequently, the column Use does not necessarily indicate clinical usage. See the list of FDA-approved therapeutic monoclonal antibodies in the monoclonal antibody therapy page.
| Name | Brand name | Type | Source | Target | Approved | Use |
|---|---|---|---|---|---|---|
| 3F8 | mab | mouse | GD2 ganglioside | neuroblastoma | ||
| Abagovomab[1] | mab | mouse | CA-125 (imitation) | ovarian cancer | ||
| Abciximab[2] | ReoPro | Fab | chimeric | CD41 (integrin alpha-IIb) | Y[3] | platelet aggregation inhibitor |
| Abituzumab[4] | mab | humanized | CD51 | cancer | ||
| Abrezekimab[5] | Fab | humanized | IL-13 | |||
| Abrilumab[6] | mab | human | integrin α4 β7 | inflammatory bowel disease, ulcerative colitis, Crohn's disease | ||
| Actoxumab[6] | mab | human | Clostridium difficile | Clostridium difficile colitis | ||
| Adalimumab[7] | Humira | mab | human | TNF-α | Y[8] | rheumatoid arthritis, Crohn's disease, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, hemolytic disease of the newborn |
| Adecatumumab[9] | mab | human | EpCAM | prostate and breast cancer | ||
| Aducanumab[10] | Aduhelm | mab | human | Amyloid beta | Y[11] | Alzheimer's disease |
| Afasevikumab[12] | mab | human | IL-17A, IL-17F | multiple sclerosis | ||
| Afelimomab[2] | F(ab')2 | mouse | TNF-α | sepsis | ||
| Alacizumab pegol[13] | F(ab')2 | humanized | VEGFR2 | cancer | ||
| Alemtuzumab[14] | Lemtrada, Campath | mab | humanized | CD52 | Y | multiple sclerosis |
| Alirocumab[15] | Praluent | mab | human | PCSK9 | Y | hypercholesterolemia |
| Altumomab pentetate | Hybri-ceaker | mab | mouse | Carcinoembryonic antigen (CEA) | colorectal cancer (diagnosis) | |
| Amatuximab[16] | mab | chimeric | mesothelin | cancer | ||
| Amivantamab | Rybrevant | BsAb | human | Epidermal growth factor receptor (EGFR), cMet | Y | non-small cell lung cancer |
| Anatumomab mafenatox[17] | Fab | mouse | Tumor-associated glycoprotein 72 (TAG-72) | non-small cell lung cancer | ||
| Andecaliximab[18] | mab | chimeric | gelatinase B | gastric cancer or gastroesophageal junction adenocarcinoma | ||
| Anetumab ravtansine[4] | mab | human | mesothelin (MSLN) | cancer | ||
| Anifrolumab[4] | Saphnelo | mab | human | IFN-α/β receptor | Y[19] | systemic lupus erythematosus |
| Ansuvimab | Ebanga | mab | human | Ebola virus glycoprotein | Y | treatment of Zaire ebolavirus (Ebola virus) |
| Anrukinzumab[13] (= IMA-638)[20] | mab | humanized | IL-13 | asthma | ||
| Apolizumab[21] | mab | humanized | HLA-DR | hematological cancers | ||
| Aprutumab ixadotin[22] | mab | human | FGFR2 | |||
| Arcitumomab[23] | CEA-Scan | Fab' | mouse | Carcinoembryonic antigen (CEA) | gastrointestinal cancers (diagnosis) | |
| Ascrinvacumab[12] | mab | human | activin receptor-like kinase 1 | cancer | ||
| Aselizumab[24] | mab | humanized | L-selectin (CD62L) | severely injured patients | ||
| Atezolizumab[25] | Tecentriq | mab | humanized | PD-L1 | Y | cancer |
| Atidortoxumab[26] | mab | human | Staphylococcus aureus alpha toxin | |||
| Atinumab[16] | mab | human | RTN4 | |||
| Atoltivimab | mab | human | part of Atoltivimab/maftivimab/odesivimab for treatment of Zaire ebolavirus (Ebola virus) | |||
| Atoltivimab/maftivimab/odesivimab | Inmazeb | mab | human | Y | treatment of Zaire ebolavirus (Ebola virus) | |
| Atorolimumab[2] | mab | human | Rhesus factor | hemolytic disease of the newborn | ||
| Avelumab[12] | Bavencio | mab | human | PD-L1 | Y | cancer |
| Azintuxizumab vedotin[27] | mab | chimeric/ humanized | CD319 | cancer | ||
| Bamlanivimab[28] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Emergency Use Authorization (EUA) when used with etesevimab[29] | COVID-19 | |
| Bapineuzumab[30] | mab | humanized | β-amyloid | Alzheimer's disease | ||
| Basiliximab[31] | Simulect | mab | chimeric | CD25 (α chain of IL-2 receptor) | Y | prevention of organ transplant rejections |
| Bavituximab[1] | mab | chimeric | phosphatidylserine | cancer, viral infections | ||
| BCD-100 | human | PD-1 | melanoma | |||
| Bebtelovimab[32] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Emergency Use Authorization (EUA)[33] | COVID-19 | |
| Bectumomab[31] | LymphoScan | Fab' | mouse | CD22 | non-Hodgkin's lymphoma (detection) | |
| Bedinvetmab[34] | Librela[35] | mab | veterinary | nerve growth factor (NGF)[35] | Y | pain associated with osteoarthritis in dogs[35] |
| Begelomab[6] | mab | mouse | DPP4 | |||
| Belantamab mafodotin[5] | Blenrep | mab | humanized | B-cell maturation antigen (BCMA) | Y | relapsed or refractory multiple myeloma |
| Belimumab[36] | Benlysta | mab | human | B-cell activating factor (BAFF) | Y | systemic lupus erythematosus without renal or CNS involvement |
| Bemarituzumab[26] | mab | humanized | FGFR2 | gastric cancer or gastroesophageal junction adenocarcinoma | ||
| Benralizumab[37] | Fasenra | mab | humanized | CD125 | Y | asthma |
| Berlimatoxumab[26] | mab | human | Staphylococcus aureus bi-component leukocidin | |||
| Bermekimab[31] | Xilonix | mab | human | IL-1α | colorectal cancer | |
| Bersanlimab[5] | mab | human | ICAM-1 | |||
| Bertilimumab[24] | mab | human | CCL11 (eotaxin-1) | severe allergic disorders | ||
| Besilesomab[38] | Scintimun | mab | mouse | Carcinoembryonic antigen (CEA)-related antigen | inflammatory lesions and metastases (detection) | |
| Bevacizumab[14] | Avastin | mab | humanized | VEGF-A | Y | metastatic cancer, retinopathy of prematurity |
| Bezlotoxumab[31] | Zinplava | mab | human | Clostridium difficile | Y | Clostridium difficile colitis |
| Biciromab[31] | FibriScint | Fab' | mouse | fibrin II, beta chain | thromboembolism (diagnosis) | |
| Bimagrumab[39] | mab | human | ACVR2B | myostatin inhibitor | ||
| Bimekizumab[10] | Bimzelx | mab | humanized | IL-17A, IL-17F, IL-17AF | Y | psoriasis |
| Birtamimab | mab | chimeric | serum amyloid A protein | amyloidosis | ||
| Bivatuzumab[31] | mab | humanized | CD44 v6 | squamous cell carcinoma | ||
| Bleselumab[12] | mab | human | CD40 | organ transplant rejection | ||
| Blinatumomab[31] | Blincyto | BiTE | mouse | CD19 | Y | pre-B Acute lymphoblastic leukemia (ALL) (CD19+) |
| Blontuvetmab[40] | Blontress | mab | veterinary | CD20 | ||
| Blosozumab[41] | mab | humanized | SOST | osteoporosis | ||
| Bococizumab[31] | mab | humanized | PCSK9 | dyslipidemia | ||
| Brazikumab[18] | mab | human | IL-23 | Crohn's disease | ||
| Brentuximab vedotin[31] | Adcentris | mab | chimeric | CD30 (TNFRSF8) | Y | Hodgkin's lymphoma, anaplastic large-cell lymphoma |
| Briakinumab[31] | mab | human | IL-12, IL-23 | psoriasis, rheumatoid arthritis, inflammatory bowel diseases, multiple sclerosis | ||
| Brodalumab[41] | Siliq | mab | human | IL-17 | Y | Plaque psoriasis |
| Brolucizumab[25] | Beovu | scFv | humanized | vascular endothelial growth factor A (VEGFA) | Y | wet age-related macular degeneration |
| Brontictuzumab[6] | mab | humanized | Notch 1 | cancer | ||
| Burosumab[18] | Crysvita | mab | human | FGF 23 | Y | X-linked hypophosphatemia |
| Cabiralizumab[40] | mab | humanized | CSF1R | metastatic pancreatic cancer | ||
| Camidanlumab tesirine[26] | mab | human | CD25 (α chain of IL-2 receptor) | B-cell Hodgkin's lymphoma, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia | ||
| Camrelizumab[22] | mab | humanized | PD-1 | hepatocellular carcinoma | ||
| Canakinumab[42] | Ilaris | mab | human | IL-1 | Y | cryopyrin-associated periodic syndrome |
| Cantuzumab mertansine[41] | mab | humanized | CanAg (a glycoform of MUC1) | colorectal cancer etc. | ||
| Cantuzumab ravtansine[41] | mab | humanized | CanAg (a glycoform of MUC1) | cancers | ||
| Caplacizumab[43] | Cablivi | sdAb | humanized | VWF | Y | thrombotic thrombocytopenic purpura, thrombosis |
| Casirivimab[28] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Yes when used with imdevimab[44][45] | COVID-19 | |
| Capromab[31] | Prostascint | mab | mouse | Glutamate carboxypeptidase II | Y | prostate cancer (detection) |
| Carlumab[16] | mab | human | MCP-1 | oncology/immune indications | ||
| Carotuximab[40] | mab | chimeric | endoglin | angiosarcoma | ||
| Catumaxomab[30] | Removab | 3funct | rat/mouse hybrid | EpCAM, CD3 | Y | ovarian cancer, malignant ascites, gastric cancer |
| cBR96-doxorubicin immunoconjugate | mab | humanized | Lewis-Y antigen | cancer | ||
| Cedelizumab[46] | mab | humanized | CD4 | prevention of organ transplant rejections, treatment of autoimmune diseases | ||
| Cemiplimab[31] | Libtayo | mab | human | PD-1 | Y | cutaneous squamous cell carcinoma |
| Cergutuzumab amunaleukin[12] | mab | humanized | IL-2 | cancer | ||
| Certolizumab pegol[42] | Cimzia | Fab' | humanized | TNF-α | Y | Crohn's disease, rheumatoid arthritis, axial spondyloarthritis, psoriasis arthritis |
| Cetrelimab[5] | mab | human | PD-1 | cancer | ||
| Cetuximab[31] | Erbitux | mab | chimeric | Epidermal growth factor receptor (EGFR) | Y | metastatic colorectal cancer and head and neck cancer |
| Cibisatamab[5] | mab | humanized | CEACAM5 | cancer | ||
| Cilgavimab[28] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Emergency Use Authorization (EUA) when used with tixagevimab[47] | COVID-19 | |
| Cirmtuzumab[31] | humanized | ROR1 | chronic lymphocytic leukemia | |||
| Citatuzumab bogatox[48] | Fab | humanized | EpCAM | ovarian cancer and other solid tumors | ||
| Cixutumumab[31] | mab | human | IGF-1 receptor (CD221) | solid tumors | ||
| Clazakizumab[49] | mab | humanized | IL-6 | rheumatoid arthritis | ||
| Clenoliximab[31] | mab | chimeric | CD4 | rheumatoid arthritis | ||
| Clivatuzumab tetraxetan[31] | hPAM4-Cide | mab | humanized | MUC1 | pancreatic cancer | |
| Codrituzumab[4] | mab | humanized | glypican 3 | cancer | ||
| Cofetuzumab pelidotin[26] | mab | humanized | PTK7 | cancer | ||
| Coltuximab ravtansine[4] | mab | chimeric | CD19 | cancer | ||
| Conatumumab[48] | mab | human | TRAIL-R2 | cancer | ||
| Concizumab[50] | Alhemo | mab | humanized | tissue factor pathway inhibitor (TFPI) | Y | bleeding with hemophilia |
| Cosfroviximab[27] | ZMapp | mab | chimeric | ebolavirus glycoprotein | Ebola virus | |
| Crenezumab[31] | mab | humanized | β-amyloid (1-40 and 1-42) | Alzheimer's disease | ||
| Crizanlizumab[22] | Adakveo | mab | humanized | selectin P | Y | sickle-cell disease |
| Crotedumab[40] | mab | human | glucagon receptor (GCGR) | diabetes | ||
| CR6261 | mab | human | Hemagglutinin (influenza) | infectious disease/influenza A | ||
| Cusatuzumab[5] | mab | humanized | CD70 | cancer | ||
| Dacetuzumab[13] | mab | humanized | CD40 | hematologic cancers | ||
| Daclizumab[51] | Zenapax | mab | humanized | CD25 (α chain of IL-2 receptor) | Y | prevention of organ transplant rejections, multiple sclerosis |
| Dalotuzumab | mab | humanized | IGF-1 receptor (CD221) | cancer etc. | ||
| Dapirolizumab pegol[52] | mab | humanized | CD154 (CD40L) | |||
| Daratumumab[53] | Darzalex | mab | human | CD38 | Y[54] | multiple myeloma |
| Dectrekumab[25] | mab | human | IL-13 | |||
| Demcizumab | mab | humanized | DLL4 | cancer | ||
| Denintuzumab mafodotin[6] | mab | humanized | CD19 | cancer | ||
| Denosumab[55] | Prolia | mab | human | RANKL | Y | osteoporosis, bone metastases etc. |
| Depatuxizumab mafodotin[22] | mab | chimeric/ humanized | EGFR | glioblastoma | ||
| Derlotuximab biotin | mab | chimeric | histone complex | recurrent glioblastoma multiforme | ||
| Detumomab | mab | mouse | B-lymphoma cell | lymphoma | ||
| Dezamizumab[22] | mab | humanized | serum amyloid P component | |||
| Dinutuximab | Unituxin | mab | chimeric | GD2 ganglioside | Y | neuroblastoma |
| Dinutuximab beta | Qarziba | mab | chimeric | GD2 ganglioside | Y | neuroblastoma |
| Diridavumab | mab | human | Hemagglutinin (influenza) | influenza A | ||
| Domagrozumab[40] | mab | humanized | GDF-8 | Duchenne muscular dystrophy | ||
| Donanemab | mab | humanized | Amyloid beta | Alzheimer's disease | ||
| Dorlimomab aritox[56] | F(ab')2 | mouse | ||||
| Dostarlimab[57] | Jemperli | mab | humanized | PCDP1 | Y | endometrial cancer |
| Drozitumab | mab | human | DR5 | cancer etc. | ||
| DS-8201 | humanized | HER2 | gastric or gastroesophageal junction adenocarcinoma | |||
| Duligotuzumab[15] | mab | humanized | ERBB3 (HER3) | testicular cancer | ||
| Dupilumab[39] | Dupixent | mab | human | IL-4Rα | Y | atopic dermatitis, asthma, nasal polyps |
| Durvalumab[25] | Imfinzi | mab | human | PD-L1 | Y | cancer |
| Dusigitumab | mab | human | IGF-2 | B-cell malignancies | ||
| Duvortuxizumab[27] | scFv | chimeric/ humanized | CD19, CD3E | cancer | ||
| Ecromeximab[21] | mab | chimeric | GD3 ganglioside | malignant melanoma | ||
| Eculizumab[21] | Soliris | mab | humanized | C5 | Y | paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome |
| Edobacomab | mab | mouse | endotoxin | sepsis caused by Gram-negative bacteria | ||
| Edrecolomab | Panorex | mab | mouse | EpCAM | colorectal carcinoma | |
| Efalizumab[7] | Raptiva | mab | humanized | LFA-1 (CD11a) | psoriasis (blocks T-cell migration) | |
| Efungumab[1] | Mycograb | scFv | human | Hsp90 | invasive Candida infection | |
| Eldelumab[4] | mab | human | CXCL10 (IP-10) | Crohn's disease, ulcerative colitis | ||
| Elezanumab[22] | mab | human | repulsive guidance molecule A (RGMA) | spinal cord injury and multiple sclerosis | ||
| Elgemtumab[25] | mab | human | ERBB3 (HER3) | cancer | ||
| Elotuzumab | Empliciti | mab | humanized | SLAMF7 | Y | multiple myeloma |
| Elsilimomab | mab | mouse | IL-6 | |||
| Emactuzumab[6] | mab | humanized | CSF1R | cancer | ||
| Emapalumab[22] | Gamifant | mab | human | IFN-γ | Y | hemophagocytic lymphohistiocytosis |
| Emibetuzumab | mab | humanized | HGFR | cancer | ||
| Emicizumab[12] | Hemlibra | BsAb | humanized | activated F9, F10 | Y | haemophilia A |
| Enapotamab vedotin[5] | mab | human | AXL | cancer | ||
| Enavatuzumab | mab | humanized | TWEAK receptor | cancer etc. | ||
| Enfortumab vedotin[4] | Padcev | mab | human | nectin-4 | Y[58] | urothelial cancer |
| Enlimomab pegol[59] | mab | mouse | ICAM-1 (CD54) | |||
| Enoblituzumab[22] | mab | humanized | CD276 | cancer | ||
| Enokizumab | mab | humanized | IL-9 | asthma | ||
| Enoticumab[15] | mab | human | DLL4 | |||
| Ensituximab | mab | chimeric | MUC5AC | cancer | ||
| Epcoritamab | Epkinly[60][61] | BiTE | humanized | CD3, CD20 | Y | diffuse large B-cell lymphoma |
| Epitumomab cituxetan[62] | mab | mouse | episialin | |||
| Epratuzumab | mab | humanized | CD22 | cancer, systemic lupus erythematosus (SLE) | ||
| Eptinezumab[22] | Vyepti | mab | humanized | calcitonin gene-related peptide | Y[63] | migraine |
| Erenumab[22] | Aimovig | mab | human | calcitonin gene-related peptide receptor (CGRP) | Y | migraine |
| Erlizumab[64] | F(ab')2 | humanized | ITGB2 (CD18) | heart attack, stroke, traumatic shock | ||
| Ertumaxomab[30] | Rexomun | 3funct | rat/mouse hybrid | HER2/neu, CD3 | Y | breast cancer etc. |
| Etaracizumab | Abegrin | mab | humanized | integrin αvβ3 | Y | melanoma, prostate cancer, ovarian cancer etc. |
| Etesevimab[28] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Emergency Use Authorization (EUA) when used with bamlanivimab[29] | COVID-19 | |
| Etigilimab[5] | mab | humanized | TIGIT | |||
| Etrolizumab[16] | mab | humanized | integrin β7 | inflammatory bowel disease | ||
| Evinacumab | Evkeeza | mab | human | angiopoietin 3 | Y | dyslipidemia |
| Evolocumab[39] | Repatha | mab | human | PCSK9 | Y | hypercholesterolemia |
| Exbivirumab[65] | mab | human | hepatitis B surface antigen | hepatitis B | ||
| Fanolesomab[17] | NeutroSpec | mab | mouse | CD15 | appendicitis (diagnosis) | |
| Faralimomab | mab | mouse | IFN receptor | |||
| Faricimab[66] | Vabysmo | mab | humanized | VEGF-A and Ang-2 | Y | angiogenesis, ocular vascular diseases |
| Farletuzumab | mab | humanized | folate receptor 1 | ovarian cancer | ||
| Fasinumab | mab | human | nerve growth factor (NGF) | acute sciatic pain | ||
| FBTA05[67][68] | Lymphomun | 3funct | rat/mouse hybrid | CD20 | chronic lymphocytic leukaemia | |
| Felvizumab | mab | humanized | respiratory syncytial virus | respiratory syncytial virus infection | ||
| Fezakinumab[69][70] | mab | human | IL-22 | rheumatoid arthritis, psoriasis | ||
| Fibatuzumab[71] | mab | humanized | ephrin receptor A3 | |||
| Ficlatuzumab | mab | humanized | Hepatocyte growth factor (HGF) | cancer etc. | ||
| Figitumumab | mab | human | IGF-1 receptor (CD221) | adrenocortical carcinoma, non-small cell lung carcinoma etc. | ||
| Firivumab[6] | mab | human | Hemagglutinin (influenza) | |||
| Flanvotumab | mab | human | TYRP1 (glycoprotein 75) | melanoma | ||
| Fletikumab | mab | human | IL-20 | rheumatoid arthritis | ||
| Flotetuzumab[5] | di-scFv | humanized | IL-3 receptor | hematological malignancies | ||
| Fontolizumab[21] | HuZAF | mab | humanized | IFN-γ | Crohn's disease etc. | |
| Foralumab[72] | mab | human | CD3E | |||
| Foravirumab[48] | mab | human | rabies virus glycoprotein | rabies (prophylaxis) | ||
| Fremanezumab[22] | Ajovy | mab | humanized | calcitonin gene-related peptide alpha and beta | Y | migraine |
| Fresolimumab[53] | mab | human | TGF-β | idiopathic pulmonary fibrosis, focal segmental glomerulosclerosis, cancer | ||
| Frovocimab[57] | mab | humanized | PCSK9 | hypercholesterolemia | ||
| Frunevetmab[73] | Solensia[74] | mab | veterinary | nerve growth factor (NGF)[74] | Y | pain associated with osteoarthritis in cats[74] |
| Fulranumab | mab | human | nerve growth factor (NGF) | pain | ||
| Futuximab[15] | mab | chimeric | Epidermal growth factor receptor (EGFR) | cancer | ||
| Galcanezumab[40] | Emgality | mab | humanized | calcitonin | Y | migraine |
| Galiximab | mab | chimeric | CD80 | B-cell lymphoma | ||
| Gancotamab | scFv | human | HER2/neu | cancer | ||
| Ganitumab | mab | human | IGF-1 receptor (CD221) | cancer | ||
| Gantenerumab[42] | mab | human | β-amyloid (1-40 and 1-42) | Alzheimer's disease | ||
| Gatipotuzumab[27] | mab | humanized | MUC1 | cancer | ||
| Gavilimomab[64] | mab | mouse | CD147 (basigin) | graft versus host disease | ||
| Gedivumab[27] | mab | human | Hemagglutinin (influenza) | |||
| Gemtuzumab ozogamicin[22] | Mylotarg | mab | humanized | CD33 | Y | acute myelogenous leukemia |
| Gevokizumab | mab | humanized | IL-1β | diabetes etc. | ||
| Gilvetmab[27] | mab | veterinary | PCDC1 | |||
| Gimsilumab[26] | mab | human | CSF2 | rheumatoid arthritis | ||
| Girentuximab[53] | Rencarex | mab | chimeric | carbonic anhydrase 9 (CA-IX) | clear cell renal cell carcinoma[75] | |
| Glembatumumab vedotin[37][76] | mab | human | GPNMB | melanoma, breast cancer | ||
| Glofitamab[60] | Columvi[77] | BsAb | humanized | CD20, CD3 | Y[77] | diffuse large B-cell lymphoma[77] |
| Golimumab[65] | Simponi | mab | human | TNF-α | Y | rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis |
| Gomiliximab | mab | chimeric | CD23 (IgE receptor) | allergic asthma | ||
| Gosuranemab | mab | humanized | tau protein | progressive supranuclear palsy | ||
| Guselkumab | Tremfya | mab | human | IL-23 | Y | psoriasis |
| Ianalumab[26] | mab | human | BAFF-R | autoimmune hepatitis | ||
| Ibalizumab[42] | Trogarzo | mab | humanized | CD4 | Y | HIV infection |
| Sintilimab | human | PD-1 | squamous cell non-small cell lung cancer | |||
| Ibritumomab tiuxetan | Zevalin | mab | mouse | CD20 | Y | non-Hodgkin's lymphoma |
| Icrucumab | mab | human | VEGFR-1 | cancer etc. | ||
| Idarucizumab[4] | Praxbind | mab | humanized | dabigatran | Y | reversal of anticoagulant effects of dabigatran |
| Ifabotuzumab[22] | mab | humanized | EPHA3 | glioblastoma multiforme[78] | ||
| Igovomab | Indimacis-125 | F(ab')2 | mouse | CA-125 | ovarian cancer (diagnosis) | |
| Iladatuzumab vedotin[26] | mab | humanized | CD79B | cancer | ||
| Imalumab[6] | mab | human | macrophage migration inhibitory factor (MIF) | cancer | ||
| Imaprelimab[5] | mab | humanized | melanoma cell adhesion molecule (MCAM) | |||
| Imciromab | Myoscint | mab | mouse | cardiac myosin | Y | cardiac imaging |
| Imdevimab[28] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Yes when used with casirivimab[44][45] | COVID-19 | |
| Imgatuzumab[15] | mab | humanized | Epidermal growth factor receptor (EGFR) | cancer | ||
| Inclacumab[43] | mab | human | selectin P | cardiovascular disease | ||
| Indatuximab ravtansine[41] | mab | chimeric | SDC1 | cancer | ||
| Indusatumab vedotin[25] | mab | human | GUCY2C | cancer | ||
| Inebilizumab[12] | Uplizna | mab | humanized | CD19 | Y[79] | cancer, systemic sclerosis, multiple sclerosis |
| Infliximab | Remicade | mab | chimeric | TNF-α | Y | rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, Crohn's disease, ulcerative colitis |
| Intetumumab[80][81] | mab | human | CD51 | solid tumors (prostate cancer, melanoma) | ||
| Inolimomab | mab | mouse | CD25 (α chain of IL-2 receptor) | graft versus host disease | ||
| Inotuzumab ozogamicin[38] | Besponsa | mab | humanized | CD22 | Y | Acute lymphoblastic leukemia (ALL) |
| Ipilimumab[55] | Yervoy | mab | human | CD152 | Y | melanoma |
| Iomab-B | mouse | CD45 | ablation of bone marrow | |||
| Iratumumab[55] | mab | human | CD30 (TNFRSF8) | Hodgkin's lymphoma | ||
| Isatuximab | Sarclisa | mab | chimeric | CD38 | Y | multiple myeloma |
| Iscalimab[5] | mab | human | CD40 | |||
| Istiratumab[26] | mab | human | IGF-1 receptor (CD221) | advanced solid tumors | ||
| Itolizumab[72] | Alzumab | mab | humanized | CD6 | Y | psoriasis |
| Ixekizumab | Taltz | mab | humanized | IL-17A | Y | autoimmune diseases |
| Keliximab | mab | chimeric | CD4 | chronic asthma | ||
| Labetuzumab[7] | CEA-Cide | mab | humanized | Carcinoembryonic antigen (CEA) | colorectal cancer | |
| Lacnotuzumab[27] | mab | humanized | CSF1, macrophage colony stimulating factor (MCSF) | cancer | ||
| Ladiratuzumab vedotin[26] | mab | humanized | LIV-1 | cancer | ||
| Lampalizumab[15] | Fab | humanized | Complement factor D (CFD) | geographic atrophy secondary to age-related macular degeneration | ||
| Lanadelumab[40] | Takhzyro | mab | human | kallikrein | Y | angioedema |
| Landogrozumab[12] | mab | humanized | GDF-8 | muscle wasting disorders | ||
| Laprituximab emtansine[40] | mab | chimeric | epidermal growth factor receptor (EGFR) | |||
| Larcaviximab[27] | mab | chimeric | ebolavirus glycoprotein | Ebola virus | ||
| Lebrikizumab | mab | humanized | IL-13 | asthma | ||
| Lecanemab[82] | Leqembi | mab | humanized | β-amyloid | Y[83] | Alzheimer's disease |
| Lemalesomab[64] | mab | mouse | NCA-90 (granulocyte antigen) | diagnostic agent | ||
| Lendalizumab[40] | mab | humanized | C5 | |||
| Lenvervimab[5] | mab | humanized | hepatitis B surfage antigen | hepatitis B | ||
| Lenzilumab[6] | mab | human | CSF2 | chronic myelomonocytic leukemia and juvenile myelomonocytic leukemia | ||
| Lerdelimumab[17] | mab | human | TGF-β2 | reduction of scarring after glaucoma surgery | ||
| Leronlimab[5] | mab | humanized | CCR5 | breast cancer, HIV | ||
| Lesofavumab[27] | mab | human | Hemagglutinin (influenza) | |||
| Letolizumab[27] | scFv | humanized | tumor necrosis factor related activation protein (TRAP) | inflammatory diseases | ||
| Lexatumumab[1] | mab | human | TRAIL-R2 | cancer | ||
| Libivirumab[65] | mab | human | hepatitis B surface antigen | hepatitis B | ||
| Lifastuzumab vedotin | mab | humanized | phosphate-sodium co-transporter | cancer | ||
| Ligelizumab[15] | mab | humanized | IGHE | severe asthma, chronic spontaneous urticaria | ||
| Loncastuximab tesirine[84][71] | Zynlonta | mab | chimeric | CD19 | Y | relapsed or refractory large B-cell lymphoma |
| Losatuxizumab vedotin[27] | mab | chimeric/ humanized | epidermal growth receptor factor (EGRF), ERBB1 HER1 | cancer | ||
| Lilotomab satetraxetan[25] | mab | mouse | CD37 | cancer | ||
| Lintuzumab | mab | humanized | CD33 | cancer | ||
| Lirilumab[15] | mab | human | KIR2D | solid and hematological cancers | ||
| Lodelcizumab[39] | mab | humanized | PCSK9 | hypercholesterolemia | ||
| Lokivetmab[25] | Cytopoint[85] | mab | veterinary | Canis lupus familiaris IL31 | Y | clinical signs of atopic dermatitis in dogs[85] |
| Lorvotuzumab mertansine | mab | humanized | CD56 | cancer | ||
| Lucatumumab[13] | mab | human | CD40 | multiple myeloma, non-Hodgkin's lymphoma, Hodgkin's lymphoma | ||
| Lulizumab pegol[6] | mab | humanized | CD28 | autoimmune diseases | ||
| Lumiliximab[9] | mab | chimeric | CD23 (IgE receptor) | chronic lymphocytic leukemia | ||
| Lumretuzumab[6] | mab | humanized | ERBB3 (HER3) | cancer | ||
| Lupartumab[22] | mab | human | ||||
| Lupartumab amadotin[22] | mab | human | LYPD3 | |||
| Lutikizumab[22] | mab | humanized | IL-1α | |||
| Maftivimab | mab | human | part of Atoltivimab/maftivimab/odesivimab for treatment of Zaire ebolavirus (Ebola virus) | |||
| Mapatumumab[30] | mab | human | TRAIL-R1 | cancer | ||
| Margetuximab | Margenza[86] | mab | humanized | HER2 | Y | breast cancer |
| Marstacimab | mab | human | tissue factor pathway inhibitor (TFPI) | bleeding with hemophilia | ||
| Maslimomab | mouse | T-cell receptor | ||||
| Mavrilimumab[37] | mab | human | GMCSF receptor α-chain | rheumatoid arthritis | ||
| Matuzumab[24] | mab | humanized | Epidermal growth factor receptor (EGFR) | colorectal, lung and stomach cancer | ||
| Mepolizumab[46] | Bosatria | mab | humanized | IL-5 | Y | asthma and white blood cell diseases |
| Metelimumab[24] | mab | human | TGF-β1 | systemic scleroderma | ||
| Milatuzumab[13] | mab | humanized | CD74 | multiple myeloma and other hematological malignancies | ||
| Minretumomab | mab | mouse | TAG-72 | tumor detection (and therapy?) | ||
| Mirikizumab[84] | Omvoh | mab | humanized | IL-23 | Y | ulcerative colitis |
| Mirvetuximab soravtansine[12] | Elahere | mab | chimeric | folate receptor alpha | Y[87] | ovarian cancer |
| Mitumomab | mab | mouse | GD3 ganglioside | small cell lung carcinoma | ||
| Modotuximab | mab | chimeric | EGFR extracellular domain III | cancer | ||
| Mogamulizumab[16] | Poteligeo | mab | humanized | CCR4 | Y | adult T-cell leukemia/lymphoma |
| Monalizumab[12] | mab | humanized | NKG2A | rheumatoid arthritis, gynecologic malignancies, and other cancers | ||
| Morolimumab[88] | mab | human | Rhesus factor | |||
| Mosunetuzumab[84] | Lunsumio | BiTE | humanized | CD3E, MS4A1, CD20 | Y[89] | follicular lymphoma |
| Motavizumab[1] | Numax | mab | humanized | respiratory syncytial virus | respiratory syncytial virus (prevention) | |
| Moxetumomab pasudotox | Lumoxiti | mab | mouse | CD22 | Y | hairy cell leukemia |
| Muromonab-CD3[90][91] | Orthoclone OKT3 | mab | mouse | CD3 | prevention of organ transplant rejections | |
| Nacolomab tafenatox | Fab | mouse | C242 antigen | colorectal cancer | ||
| Namilumab[16] | mab | human | CSF2 | |||
| Naptumomab estafenatox[92] | Fab | mouse | 5T4 | non-small cell lung carcinoma, renal cell carcinoma | ||
| Naratuximab emtansine[40] | mab | chimeric | CD37 | |||
| Narnatumab | mab | human | MST1R (aka RON) | cancer | ||
| Natalizumab[88] | Tysabri | mab | humanized | integrin α4 | Y | multiple sclerosis, Crohn's disease |
| Navicixizumab[40] | mab | chimeric/ humanized | DLL4 and VEGFA | cancer | ||
| Navivumab[12] | mab | human | Hemagglutinin (influenza) | |||
| Naxitamab | Danyelza | humanized | c-Met | Y | high-risk neuroblastoma and refractory osteomedullary disease | |
| Nebacumab | mab | human | endotoxin | sepsis | ||
| Necitumumab[93] | Portrazza | mab | human | Epidermal growth factor receptor (EGFR) | Y | non-small cell lung carcinoma |
| Nemolizumab[25] | mab | humanized | IL-31 receptor A | eczema[94] | ||
| NEOD001 | humanized | amyloid | primary systemic amyloidosis | |||
| Nerelimomab | mab | mouse | TNF-α | |||
| Nesvacumab | mab | human | angiopoietin 2 | cancer | ||
| Netakimab[5] | Efleira | mab | chimeric | IL-17A | plaque psoriasis | |
| Nimotuzumab[55][95] | BioMab-EGFR, Theracim, Theraloc | mab | humanized | epidermal growth factor receptor (EGFR) | Y | squamous cell carcinoma, head and neck cancer, nasopharyngeal cancer, glioma |
| Nirsevimab | mab | human | RSV fusion glycoprotein | respiratory syncytial virus | ||
| Nivolumab[6] | Opdivo | mab | human | PD-1 | Y | cancer |
| Nofetumomab merpentan | Verluma | Fab | mouse | cancer (diagnosis) | ||
| Obiltoxaximab | Anthim | mab | chimeric | Bacillus anthracis anthrax | Y | Bacillus anthracis spores |
| Obinutuzumab | Gazyva | mab | humanized | CD20 | Y | chronic lymphatic leukemia |
| Ocaratuzumab | mab | humanized | CD20 | cancer | ||
| Ocrelizumab[96] | Ocrevus | mab | humanized | CD20 | Y | multiple sclerosis |
| Odesivimab | mab | human | part of Atoltivimab/maftivimab/odesivimab for treatment of Zaire ebolavirus (Ebola virus) | |||
| Odulimomab | mab | mouse | LFA-1 (CD11a) | prevention of organ transplant rejections, immunological diseases | ||
| Ofatumumab[30] | Arzerra, Kesimpta[97] | mab | human | CD20 | Y | chronic lymphocytic leukemia, multiple sclerosis[97] |
| Olaratumab | Lartruvo | mab | human | PDGFRA | Y | cancer |
| Oleclumab[27] | mab | human | 5'-nucleotidase | pancreatic and colorectal cancer | ||
| Olendalizumab[27] | mab | humanized | complement C5a | systemic lupus erythematosus, lupus nephritis, acute graft-versus-hose disease | ||
| Olokizumab[72] | mab | humanized | IL-6 | rheumatoid arthritis | ||
| Omalizumab[64] | Xolair | mab | humanized | IgE Fc region | Y | allergic asthma, chronic spontaneous urticaria |
| Omburtamab[57] | mab | mouse | CD276 | cancer | ||
| OMS721 | human | MASP-2 | atypical hemolytic uremic syndrome | |||
| Onartuzumab | Fab | humanized | human scatter factor receptor kinase | cancer | ||
| Ontuxizumab | mab | chimeric/ humanized | TEM1 | cancer | ||
| Onvatilimab[5] | mab | human | VISTA (protein) (VSIR) | |||
| Opicinumab | mab | human | LINGO-1 | multiple sclerosis | ||
| Oportuzumab monatox[93] | Vicinium | scFv | humanized | EpCAM | bladder cancer | |
| Oregovomab[17] | OvaRex | mab | mouse | CA-125 | ovarian cancer | |
| Orticumab[15] | mab | human | oxLDL | |||
| Otelixizumab[13] | mab | chimeric/ humanized | CD3 | diabetes mellitus type 1 | ||
| Otilimab | mab | human | GMCSF | osteoarthritis, rheumatoid arthritis | ||
| Otlertuzumab | mab | humanized | CD37 | cancer | ||
| Oxelumab[41] | mab | human | OX-40 | asthma | ||
| Ozanezumab | mab | humanized | NOGO-A | ALS and multiple sclerosis | ||
| Ozoralizumab[41] | mab | humanized | TNF-α | inflammation | ||
| Pagibaximab[30] | mab | chimeric | lipoteichoic acid | sepsis (Staphylococcus) | ||
| Palivizumab | Synagis, Abbosynagis | mab | humanized | F protein of respiratory syncytial virus | Y | respiratory syncytial virus (prevention) |
| Pamrevlumab[12] | mab | human | connective tissue growth factor (CTGF) | idiopathic pulmonary fibrosis (IPF), pancreatic cancer | ||
| Panitumumab[65] | Vectibix | mab | human | epidermal growth factor receptor (EGFR) | Y | colorectal cancer |
| Pankomab | mab | humanized | tumor specific glycosylation of MUC1 | ovarian cancer | ||
| Panobacumab[93] | mab | human | Pseudomonas aeruginosa | Pseudomonas aeruginosa infection | ||
| Parsatuzumab[15] | mab | humanized | EGFL7 | cancer | ||
| Pascolizumab[21] | mab | humanized | IL-4 | asthma | ||
| Pasotuxizumab[6] | mab | chimeric/ humanized | folate hydrolase | cancer | ||
| Pateclizumab[41] | mab | humanized | lymphotoxin alpha (LTA) | TNF | ||
| Patritumab[43] | mab | human | ERBB3 (HER3) | cancer | ||
| PDR001 | humanized | PD-1 | melanoma | |||
| Pembrolizumab[52] | Keytruda | mab | humanized | PD-1 | Y[98] | melanoma and other cancers |
| Pemtumomab | Theragyn | mouse | MUC1 | cancer | ||
| Perakizumab[15] | mab | humanized | IL-17A | arthritis | ||
| Pertuzumab | Perjeta | mab | humanized | HER2/neu | Y | cancer |
| Pexelizumab[17] | scFv | humanized | C5 | reduction of side effects of cardiac surgery | ||
| Pidilizumab[39] | mab | humanized | PD-1 | cancer and infectious diseases | ||
| Pinatuzumab vedotin[39] | mab | humanized | CD22 | cancer | ||
| Pintumomab | mab | mouse | adenocarcinoma antigen | adenocarcinoma (imaging) | ||
| Placulumab[15] | mab | human | TNF | pain and inflammatory diseases | ||
| Pozelimab[34] | Veopoz | mab | human | C5 | Y | CHAPLE disease |
| Prezalumab[40] | mab | human | TNF | |||
| Plozalizumab[12] | mab | humanized | CCR2 | diabetic nephropathy and arteriovenous graft patency | ||
| Pogalizumab[40] | mab | humanized | tumor necrosis factor receptor (TNFR) superfamily member 4 | |||
| Polatuzumab vedotin[50][4] | Polivy | mab | humanized | CD79B | Y | diffuse large B-cell lymphoma |
| Ponezumab | mab | humanized | β-amyloid | Alzheimer's disease | ||
| Porgaviximab[27] | mab | chimeric | Zaire ebolavirus glycoprotein | Ebola virus disease | ||
| Prasinezumab[26] | mab | humanized | Alpha-synuclein | Parkinson's disease | ||
| Prezalizumab[40] | mab | humanized | inducible T-cell co-stimulatory ligand (ICOSL) | |||
| Priliximab | mab | chimeric | CD4 | Crohn's disease, multiple sclerosis | ||
| Pritoxaximab[39] | mab | chimeric | E. coli shiga toxin type-1 | |||
| Pritumumab | mab | human | vimentin | brain cancer | ||
| PRO 140 | humanized | CCR5 | HIV infection | |||
| Quilizumab[43] | mab | humanized | IGHE | asthma | ||
| Racotumomab[93] | Vaxira | mab | mouse | NGNA ganglioside | Y | non-small cell lung cancer |
| Radretumab[16] | mab | human | fibronectin extra domain-B | cancer | ||
| Rafivirumab[48] | mab | human | rabies virus glycoprotein | rabies (prophylaxis) | ||
| Ralpancizumab | mab | humanized | PCSK9 | dyslipidemia | ||
| Ramucirumab | Cyramza | mab | human | VEGFR2 | Y | solid tumors |
| Ranevetmab | mab | veterinary | NGF | osteoarthritis in dogs | ||
| Ranibizumab[9] | Lucentis | Fab | humanized | VEGF-A | Y | macular degeneration (wet form) |
| Raxibacumab[38] | mab | human | anthrax toxin, protective antigen | Y | anthrax (prophylaxis and treatment) | |
| Ravagalimab[5] | mab | humanized | CD40 | Crohn's disease | ||
| Ravulizumab[26] | Ultomiris | mab | humanized | C5 | Y | paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome |
| Refanezumab | mab | humanized | myelin-associated glycoprotein | recovery of motor function after stroke | ||
| Regavirumab | mab | human | cytomegalovirus glycoprotein B | cytomegalovirus infection | ||
| Regdanvimab[28] | Regkirona[99] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Y[99] | COVID-19 |
| Relatlimab | mab | human | LAG3 | melanoma | ||
| Remtolumab[22] | mab | human | IL-17A, TNF | |||
| Reslizumab[7] | Cinqair | mab | humanized | IL-5 | Y | inflammations of the airways, skin and gastrointestinal tract |
| Retifanlimab[60] | Zynyz[100] | mab | humanized | PD-1 | Y | Merkel cell carcinoma |
| Rilotumumab | mab | human | hepatocyte growth factor (HGF) | solid tumors | ||
| Rinucumab | mab | human | PDGFRB | neovascular age-related macular degeneration | ||
| Risankizumab[12] | Skyrizi | mab | humanized | IL-23A | Y | Crohn's disease, psoriasis, psoriatic arthritis, and asthma |
| Rituximab | MabThera, Rituxan | mab | chimeric | CD20 | Y[101] | lymphomas, leukemias, some autoimmune disorders |
| Rivabazumab pegol[12] | mab | humanized | Pseudomonas aeruginosa type III secretion system | |||
| Robatumumab | mab | human | IGF-1 receptor (CD221) | cancer | ||
| Rmab | RabiShield | human | rabies virus G glycoprotein | Y | post-exposure prophylaxis of rabies | |
| Roledumab[72] | mab | human | RHD (gene) (RHD) | Rh disease | ||
| Romilkimab[5] | mab | chimeric/ humanized | IL-13 | |||
| Romosozumab | Evenity | mab | humanized | sclerostin | Y | osteoporosis |
| Rontalizumab[53] | mab | humanized | IFN-α | systemic lupus erythematosus | ||
| Rosmantuzumab[22] | mab | humanized | root plate-specific spondin 3 | cancer | ||
| Rovalpituzumab tesirine[12] | mab | humanized | DLL3 | small cell lung cancer | ||
| Rovelizumab[46] | LeukArrest | mab | humanized | CD11, CD18 | Y | haemorrhagic shock etc. |
| Rozanolixizumab[22] | Rystiggo | mab | chimeric/ humanized | FCGRT | Y[102] | myasthenia gravis |
| Ruplizumab[14] | Antova | mab | humanized | CD154 (CD40L) | Y | rheumatic diseases |
| SA237 | humanized | IL-6 receptor | neuromyelitis optica and neuromyelitis optica spectrum disorders | |||
| Sacituzumab govitecan[22] | Trodelvy | mab | humanized | TROP-2 | Y[103] | triple-negative breast cancer |
| Samalizumab[41] | mab | humanized | CD200 | cancer | ||
| Samrotamab vedotin[5] | mab | chimeric/ humanized | LRRC15 | cancer | ||
| Sarilumab[43] | Kevzara | mab | human | IL-6 | Y | rheumatoid arthritis, ankylosing spondylitis |
| Satralizumab[73] | Enspryng | mab | humanized | IL-6 receptor | Y | neuromyelitis optica |
| Satumomab pendetide | mab | mouse | TAG-72 | cancer (diagnosis) | ||
| Secukinumab | Cosentyx | mab | human | IL-17A | Y | uveitis, rheumatoid arthritis psoriasis |
| Selicrelumab[27] | mab | human | CD40 | |||
| Seribantumab[39] | mab | human | ERBB3 (HER3) | cancer | ||
| Setoxaximab[39] | mab | chimeric | E. coli shiga toxin type-2 | |||
| Setrusumab[26] | mab | human | sclerostin (SOST) | |||
| Sevirumab | human | cytomegalovirus | cytomegalovirus infection | |||
| Sibrotuzumab | mab | humanized | FAP (gene) (FAP) | cancer | ||
| SGN-CD19A | mab | humanized | CD19 | acute lymphoblastic leukemia and B-cell non-Hodgkin lymphoma | ||
| SHP647 | human | mucosal addressin cell adhesion molecule | Crohn's disease | |||
| Sifalimumab[16] | mab | human | IFN-α | systemic lupus erythematosus (SLE), dermatomyositis, polymyositis | ||
| Siltuximab | Sylvant | mab | chimeric | IL-6 | Y | cancer |
| Simtuzumab[15] | mab | humanized | LOXL2 | fibrosis | ||
| Siplizumab[21] | mab | humanized | CD2 | psoriasis, graft-versus-host disease (prevention) | ||
| Sirtratumab vedotin[26] | mab | human | SLITRK6 | cancer | ||
| Sirukumab | mab | human | IL-6 | rheumatoid arthritis | ||
| Sofituzumab vedotin | mab | humanized | CA-125 | ovarian cancer | ||
| Solanezumab[93] | mab | humanized | β-amyloid | Alzheimer's disease | ||
| Solitomab[43] | BiTE | mouse | EpCAM | gastrointestinal, lung, and other cancers | ||
| Sonepcizumab[104] | humanized | sphingosine-1-phosphate | choroidal and retinal neovascularization | |||
| Sontuzumab[95] | mab | humanized | episialin | |||
| Sotrovimab[28] | Xevudy | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Y[105][106] | COVID-19 |
| Spartalizumab[26] | mab | humanized | PD-1 | melanoma | ||
| Spesolimab[107] | Spevigo | mab | humanized | Interleukin 36 receptor (IL1RL2/IL1RAP) | Y | generalized pustular psoriasis (GPP) |
| Stamulumab[96] | mab | human | myostatin | muscular dystrophy | ||
| Sulesomab | LeukoScan | Fab' | mouse | NCA-90 (granulocyte antigen) | osteomyelitis (imaging) | |
| Suptavumab[22] | mab | human | RSVFR | medically attended lower respiratory disease | ||
| Sutimlimab[66] | Enjaymo | mab | chimeric/ humanized | complement component 1s (C1s) | Y | cold agglutinin disease |
| Suvizumab[37] | mab | humanized | HIV-1 | viral infections | ||
| Suvratoxumab[27] | mab | human | Staphylococcus aureus alpha toxin | nosocomial pneumonia | ||
| Tabalumab[41] | mab | human | B-cell activating factor (BAFF) | B-cell cancers | ||
| Tacatuzumab tetraxetan | AFP-Cide | mab | humanized | alpha-fetoprotein | cancer | |
| Tadocizumab[95] | Fab | humanized | integrin αIIbβ3 | percutaneous coronary intervention | ||
| Tafasitamab[46] | Monjuvi | mab | humanized (from mouse) | CD19 | Y | relapsed or refractory diffuse large B-cell lymphoma |
| Talacotuzumab[26] | mab | humanized | CD123 | leukemia etc. | ||
| Talizumab[36] | mab | humanized | IgE | allergic reaction | ||
| Talquetamab | Talvey | BsAb | humanized | GPRC5D, CD3 | Y | relapsed or refractory multiple myeloma |
| Tamtuvetmab[40] | Tactress | mab | veterinary | CD52 | ||
| Tanezumab[48] | mab | humanized | nerve growth factor (NGF) | pain | ||
| Taplitumomab paptox[64] | mab | mouse | CD19 | cancer | ||
| Tarextumab | mab | human | Notch receptor | cancer | ||
| Tavolimab | mab | chimeric/ humanized | CD134 | cancer | ||
| Teclistamab | Tecvayli[34] | BsAb | human | B-cell maturation antigen (BCMA), CD3 | Y[108][109] | relapsed or refractory multiple myeloma |
| Tefibazumab[38] | Aurexis | mab | humanized | clumping factor A | Staphylococcus aureus infection | |
| Telimomab aritox | Fab | mouse | ||||
| Telisotuzumab[22] | mab | humanized | HGFR | cancer | ||
| Telisotuzumab vedotin[22] | mab | humanized | HGFR | cancer | ||
| Tenatumomab[13] | mab | mouse | tenascin C | cancer | ||
| Teneliximab[21] | mab | chimeric | CD40 | autoimmune diseases and prevention of organ transplant rejection | ||
| Teplizumab[42] | Tzield | mab | humanized | CD3 | Y | diabetes mellitus type 1 |
| Tepoditamab[5] | mab | human | dendritic cell-associated lectin 2 | cancer | ||
| Teprotumumab | Tepezza | mab | human | IGF-1 receptor (CD221) | Y | thyroid eye disease |
| Tesidolumab[25] | mab | human | C5 | |||
| Tetulomab | mab | humanized | CD37 | cancer[110] | ||
| Tezepelumab[12] | Tezspire | mab | human | thymic stromal lymphopoietin (TSLP) | Y[111] | asthma |
| TGN1412 | humanized | CD28 | chronic lymphocytic leukemia, rheumatoid arthritis | |||
| Tibulizumab[26] | mab | humanized | B-cell activating factor (BAFF) | autoimmune disorders | ||
| Tildrakizumab | Ilumya | mab | humanized | IL-23 | Y | immunologically mediated inflammatory disorders |
| Tigatuzumab[13] | mab | humanized | TRAIL-R2 | cancer | ||
| Timigutuzumab[27] | mab | humanized | HER2 | cancer | ||
| Timolumab[40] | mab | human | AOC3 | |||
| tiragolumab[26] | mab | human | ||||
| Tiragotumab[26] | mab | human | TIGIT | cancer | ||
| Tislelizumab[26] | mab | humanized | PCDC1, CD279 | non-small cell lung cancer | ||
| Tisotumab vedotin[12] | Tivdak | mab | human | coagulation factor III | Y | relapsed or refractory cervical cancer[112][113] |
| Tixagevimab[28] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Emergency Use Authorization (EUA) when used with cilgavimab[47] | COVID-19 | |
| TNX-650 | humanized | IL-13 | Hodgkin's lymphoma | |||
| Tocilizumab[9] | Actemra, RoActemra | mab | humanized | IL-6 receptor | Y[114] | rheumatoid arthritis |
| Tomuzotuximab[27] | mab | humanized | Epidermal growth factor receptor (EGFR), HER1 | cancer | ||
| Toralizumab[21] | mab | humanized | CD154 (CD40L) | rheumatoid arthritis, lupus nephritis etc. | ||
| Tosatoxumab[4] | mab | human | Staphylococcus aureus | |||
| Tositumomab | Bexxar | mouse | CD20 | Y | follicular lymphoma | |
| Tovetumab | mab | human | PDGFRA | cancer | ||
| Tralokinumab[115] | Adtralza, Adbry[116] | mab | human | IL-13 | Y[116] | atopic dermatitis |
| Trastuzumab | Herceptin | mab | humanized | HER2/neu | Y | breast cancer |
| Trastuzumab duocarmazine[22] | Kadcyla | mab | humanized | HER2/neu | Y | breast cancer |
| Trastuzumab emtansine | Kadcyla | mab | humanized | HER2/neu | Y | breast cancer |
| TRBS07[117] | Ektomab | 3funct | GD2 ganglioside | melanoma | ||
| Tregalizumab[16] | mab | humanized | CD4 | |||
| Tremelimumab[118] | Imjudo | mab | human | CTLA-4 | Y[119] | hepatocellular carcinoma |
| Trevogrumab | mab | human | growth differentiation factor 8 | muscle atrophy due to orthopedic disuse and sarcopenia | ||
| Tucotuzumab celmoleukin[55][95] | mab | humanized | EpCAM | cancer | ||
| Tuvirumab | human | hepatitis B virus | chronic hepatitis B | |||
| Ublituximab[120] | Briumvi | mab | chimeric | CD20 | Y | multiple sclerosis, chronic lymphocytic leukemia |
| Ulocuplumab[52] | mab | human | CXCR4 (CD184) | hematologic malignancies | ||
| Urelumab[16] | mab | human | 4-1BB (CD137) | cancer etc. | ||
| Urtoxazumab[9] | mab | humanized | Escherichia coli | diarrhoea caused by E. coli | ||
| Ustekinumab[48] | Stelara | mab | human | IL-12, IL-23 | Y | multiple sclerosis, psoriasis, psoriatic arthritis |
| Utomilumab[22] | mab | human | 4-1BB (CD137) | diffuse large B-cell lymphoma | ||
| Vadastuximab talirine[12] | mab | chimeric | CD33 | Acute myeloid leukemia | ||
| Vanalimab[5] | mab | humanized | CD40 | |||
| Vandortuzumab vedotin[25] | mab | humanized | STEAP1 | cancer | ||
| Vantictumab | mab | human | Frizzled receptor | cancer | ||
| Vanucizumab[6] | mab | humanized | angiopoietin 2 | cancer | ||
| Vapaliximab[21] | mab | chimeric | AOC3 (VAP-1) | |||
| Varisacumab[27] | mab | human | VEGF-A | angiogenesis | ||
| Varlilumab[6] | mab | human | CD27 | solid tumors and hematologic malignancies | ||
| Vatelizumab[41] | mab | humanized | ITGA2 (CD49b) | |||
| Vedolizumab[93] | Entyvio | mab | humanized | integrin α4 β7 | Y[121] | Crohn's disease, ulcerative colitis |
| Veltuzumab[13] | mab | humanized | CD20 | non-Hodgkin's lymphoma | ||
| Vepalimomab | mab | mouse | AOC3 (VAP-1) | inflammation | ||
| Vesencumab[16] | mab | human | NRP1 | solid malignancies | ||
| Vilobelimab[82] | Gohibic | mab | chimeric | C5a receptor (C5a) | Emergency Use Authorization (EUA)[122] | COVID-19 |
| Visilizumab[64] | Nuvion | mab | humanized | CD3 | Crohn's disease, ulcerative colitis | |
| Vobarilizumab[40] | scFv | humanized | IL-6 receptor | inflammatory autoimmune diseases | ||
| Volociximab[30] | mab | chimeric | integrin α5β1 | solid tumors | ||
| Vonlerolizumab[27] | mab | humanized | CD134 | cancer | ||
| Vopratelimab[5] | mab | humanized | CD278, aka ICOS | |||
| Vorsetuzumab mafodotin | mab | humanized | CD70 | cancer | ||
| Votumumab | HumaSPECT | mab | human | tumor antigen CTAA16.88 | colorectal tumors | |
| Vunakizumab[22] | mab | humanized | IL-17A | |||
| Xentuzumab[40] | mab | humanized | IGF-1, IGF-2 | ? | ||
| XMAB-5574 | humanized | CD19 | diffuse large B-cell lymphoma | |||
| Zalutumumab[30] | mab | human | Epidermal growth factor receptor (EGFR) | squamous cell carcinoma of the head and neck | ||
| Zanolimumab[38] | mab | human | CD4 | rheumatoid arthritis, psoriasis, T-cell lymphoma | ||
| Zatuximab[15] | mab | chimeric | HER1 | cancer | ||
| Zenocutuzumab[26] | mab | humanized | ERBB3 (HER3) | cancer | ||
| Ziralimumab[64] | mab | human | CD147 (basigin) | |||
| Zolbetuximab[26] | mab | chimeric | Claudin 18 Isoform 2 | gastric cancer, gastrointestinal adenocarcinoma and pancreatic cancer | ||
| Zolimomab aritox | mab | mouse | CD5 | systemic lupus erythematosus, graft-versus-host disease |
References
- World Health Organization (2007). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 57". WHO Drug Information. 21 (1). hdl:10665/74004.
- World Health Organization (1998). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 80" (PDF). WHO Drug Information. 12 (4). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- "ReoPro Abciximab". DailyMed. 4 May 2020. Archived from the original on 6 September 2022. Retrieved 28 June 2022.
- World Health Organization (2014). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 71". WHO Drug Information. 28 (1). hdl:10665/331151.
- World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 118" (PDF). WHO Drug Information. 31 (4). Archived (PDF) from the original on 15 August 2020. Retrieved 5 October 2020.
- World Health Organization (2014). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 111" (PDF). WHO Drug Information. 28 (2). Archived (PDF) from the original on 15 August 2020. Retrieved 5 October 2020.
- World Health Organization (2001). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 85" (PDF). WHO Drug Information. 15 (2). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- "Humira- adalimumab kit Humira- adalimumab injection, solution". DailyMed. Archived from the original on 21 June 2020. Retrieved 18 February 2020.
- World Health Organization (2004). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 90" (PDF). WHO Drug Information. 18 (1). Archived (PDF) from the original on 15 August 2020. Retrieved 23 January 2020.
- World Health Organization (2014). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 72". WHO Drug Information. 28 (3). hdl:10665/331112.
- "Aduhelm- aducanumab injection, solution". DailyMed. Archived from the original on 14 June 2021. Retrieved 14 June 2021.
- World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 75". WHO Drug Information. 30 (1). hdl:10665/331046.
- World Health Organization (2007). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 98" (PDF). WHO Drug Information. 21 (4). Archived (PDF) from the original on 3 March 2016. Retrieved 5 October 2020.
- World Health Organization (2000). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 83" (PDF). WHO Drug Information. 14 (2). Archived (PDF) from the original on 15 August 2020. Retrieved 23 January 2020.
- World Health Organization (2012). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 107" (PDF). WHO Drug Information. 26 (2). Archived (PDF) from the original on 7 August 2020. Retrieved 23 January 2020.
- World Health Organization (2010). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 104" (PDF). WHO Drug Information. 24 (4). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2002). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 86" (PDF). WHO Drug Information. 16 (1). Archived (PDF) from the original on 11 August 2020. Retrieved 23 January 2020.
- World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 115" (PDF). WHO Drug Information. 30 (2). Archived (PDF) from the original on 5 February 2018. Retrieved 5 October 2020.
- "Saphnelo- anifrolumab injection, solution". DailyMed. Archived from the original on 12 August 2021. Retrieved 11 August 2021.
- "Wyeth.com | Complete Project Listing". Wyeth. 2008. Archived from the original on 12 June 2008. Retrieved 19 November 2008.
- World Health Organization (2002). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 87" (PDF). WHO Drug Information. 16 (2). Archived (PDF) from the original on 7 August 2020. Retrieved 23 January 2020.
- World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 77". WHO Drug Information. 31 (1). hdl:10665/330984.
- World Health Organization (1995). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 74" (PDF). WHO Drug Information. 9 (4). Archived (PDF) from the original on 7 August 2020. Retrieved 24 January 2020.
- World Health Organization (2003). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 88" (PDF). WHO Drug Information. 17 (1). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2014). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 112" (PDF). WHO Drug Information. 28 (4). Archived (PDF) from the original on 28 August 2021. Retrieved 5 October 2020.
- World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 117" (PDF). WHO Drug Information. 31 (2). Archived (PDF) from the original on 15 August 2020. Retrieved 5 October 2020.
- World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 116" (PDF). WHO Drug Information. 30 (4). Archived (PDF) from the original on 7 August 2020. Retrieved 5 October 2020.
- World Health Organization (2021). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 85". WHO Drug Information. 35 (1). hdl:10665/340684.
- "Fact Sheet For Health Care Providers Emergency Use Authorization (EUA) Of Bamlanivimab and Etesevimab" (PDF). U.S. Food and Drug Administration (FDA). Archived from the original on 18 March 2021. Retrieved 18 March 2021.
- World Health Organization (2008). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 93" (PDF). WHO Drug Information. 19 (2). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2019). International Nonproprietary Names (INN) for biological and biotechnological substances (PDF). World Health Organization (WHO). WHO/EMP/RHT/TSN/2019.1. Archived (PDF) from the original on 19 January 2022. Retrieved 22 January 2020.
- World Health Organization (2021). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 126 – COVID-19 (special edition)". WHO Drug Information. 35 (4): 1130–1. Archived from the original on 13 February 2022. Retrieved 13 February 2022.
- "Fact Sheet For Health Care Providers Emergency Use Authorization (EUA) Of Bebtelovimab" (PDF). U.S. Food and Drug Administration (FDA). Archived from the original on 11 February 2022. Retrieved 12 February 2022.
- World Health Organization (2019). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 82". WHO Drug Information. 33 (3). hdl:10665/330879.
- "Zoetis Announces U.S. FDA Approval of Librela (bedinvetmab injection) to Control Osteoarthritis (OA) Pain in Dogs" (Press release). Zoetis. 5 May 2023. Archived from the original on 6 May 2023. Retrieved 13 May 2023 – via Business Wire.
- World Health Organization (2003). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 89" (PDF). WHO Drug Information. 17 (3).
- World Health Organization (2009). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 102" (PDF). WHO Drug Information. 23 (4). Archived (PDF) from the original on 11 August 2020. Retrieved 23 January 2020.
- World Health Organization (2004). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 92" (PDF). WHO Drug Information. 18 (4). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2012). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 108" (PDF). WHO Drug Information. 26 (4). Archived (PDF) from the original on 4 March 2016. Retrieved 5 October 2020.
- World Health Organization (2015). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 114" (PDF). WHO Drug Information. 29 (4). Archived (PDF) from the original on 7 August 2020. Retrieved 5 October 2020.
- World Health Organization (2011). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 105" (PDF). WHO Drug Information. 25 (2). Archived (PDF) from the original on 11 August 2020. Retrieved 23 January 2020.
- World Health Organization (2007). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 97" (PDF). WHO Drug Information. 21 (2). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2011). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 106" (PDF). WHO Drug Information. 25 (4). Archived (PDF) from the original on 15 August 2020. Retrieved 23 January 2020.
- "Ronapreve EPAR". European Medicines Agency. 10 November 2021. Archived from the original on 13 November 2021. Retrieved 12 November 2021.
- "Japan becomes first country to approve Ronapreve (casirivimab and imdevimab) for the treatment of mild to moderate COVID-19". Roche (Press release). 20 July 2021. Archived from the original on 24 July 2021. Retrieved 29 August 2021.
- World Health Organization (1999). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 81" (PDF). WHO Drug Information. 13 (2). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- "Coronavirus (COVID-19) Update: FDA Authorizes New Long-Acting Monoclonal Antibodies for Pre-exposure Prevention of COVID-19 in Certain Individuals". U.S. Food and Drug Administration (FDA) (Press release). 8 December 2021. Archived from the original on 28 April 2022. Retrieved 9 December 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - World Health Organization (2008). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 99" (PDF). WHO Drug Information. 22 (2). Archived (PDF) from the original on 25 October 2021. Retrieved 5 October 2020.
- "BMS Shells Out $85M Up Front for Alder's Mid-Stage Rheumatoid Arthritis Antibody". Genetic Engineering & Biotechnology News. 10 November 2009. Archived from the original on 13 November 2009. Retrieved 11 November 2009.
- World Health Organization (2013). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 70". WHO Drug Information. 27 (3). hdl:10665/331167.
- World Health Organization (1997). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 78" (PDF). WHO Drug Information. 11 (4). Archived (PDF) from the original on 7 August 2020. Retrieved 23 January 2020.
- World Health Organization (2013). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 110" (PDF). WHO Drug Information. 27 (4). Archived (PDF) from the original on 7 August 2020. Retrieved 5 October 2020.
- World Health Organization (2009). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 101" (PDF). WHO Drug Information. 23 (2). Archived (PDF) from the original on 11 August 2020. Retrieved 23 January 2020.
- "Darzalex- daratumumab injection, solution, concentrate Darzalex IV- daratumumab injection, solution, concentrate". DailyMed. Archived from the original on 19 December 2021. Retrieved 18 December 2021.
- World Health Organization (2005). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 94" (PDF). WHO Drug Information. 19 (4). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (1991). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 66" (PDF). WHO Drug Information. 5 (4). Archived (PDF) from the original on 15 August 2020. Retrieved 23 January 2020.
- World Health Organization (2018). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 119" (PDF). WHO Drug Information. 32 (2). Archived (PDF) from the original on 11 August 2020. Retrieved 5 October 2020.
- "Padcev ejfv- enfortumab vedotin injection, powder, lyophilized, for solution". DailyMed. Archived from the original on 11 December 2021. Retrieved 18 December 2021.
- World Health Organization (1997). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 77" (PDF). WHO Drug Information. 11 (2). Archived (PDF) from the original on 11 August 2020. Retrieved 23 January 2020.
- World Health Organization (2020). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 83". WHO Drug Information. 34 (1). hdl:10665/339768.
- World Health Organization (2022). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 88". WHO Drug Information. 36 (3). hdl:10665/363551.
- World Health Organization (2004). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 51" (PDF). WHO Drug Information. 18 (1). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- "Vyepti- eptinezumab-jjmr injection". DailyMed. Archived from the original on 28 September 2021. Retrieved 27 September 2021.
- World Health Organization (2000). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 84" (PDF). WHO Drug Information. 14 (4). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2004). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 91" (PDF). WHO Drug Information. 18 (2). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2018). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 80". WHO Drug Information. 32 (3). hdl:10665/330907.
- Buhmann, R; Simoes, B; Stanglmaier, M; Yang, T; Faltin, M; Bund, D; Lindhofer, H; Kolb, HJ; et al. (2008). "Immunotherapy of recurrent B–cell malignancies after allo SCT with Bi20 (FBTA05), a trifunctional anti-CD3 x anti-CD20 antibody and donor lymphocyte infusion". Bone Marrow Transplantation. 43 (5): 383–397. doi:10.1038/bmt.2008.323. PMID 18850012.
- Boehrer, S; Schroeder, Petra; Mueller, Tina; Atz, Judith; Chow, Kai Uwe; et al. (2011). "Cytotoxic effects of the trifunctional bispecific antibody FBTA05 in ex-vivo cells of chronic lymphocytic leukaemia depend on immune-mediated mechanisms". Anti-Cancer Drugs. 12 (10): 3085–3091. doi:10.1097/CAD.0b013e328344887f. PMID 21637160. S2CID 29327089.
- Clinical trial number NCT00883896 for "Study to Evaluate the Safety and Efficacy of ILV-094 in Subjects With Rheumatoid Arthritis" at ClinicalTrials.gov
- Clinical trial number NCT00563524 for "Study Evaluating the Safety and Tolerability of ILV-094 in Subjects With Psoriasis" at ClinicalTrials.gov
- World Health Organization (2015). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 113" (PDF). WHO Drug Information. 29 (2). Archived (PDF) from the original on 4 November 2021. Retrieved 5 October 2020.
- World Health Organization (2011). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 103" (PDF). WHO Drug Information. Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78". WHO Drug Information. 31 (3). hdl:10665/330961.
- "FDA Approves Novel Treatment to Control Pain in Cats with Osteoarthritis, First Monoclonal Antibody Drug for Use in Any Animal Species". U.S. Food and Drug Administration (FDA) (Press release). 13 January 2022. Archived from the original on 13 January 2022. Retrieved 14 January 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Prometheus Obtains Exclusive US Commercialization Rights for RENCAREX®" (Press release). Archived from the original on 7 July 2017. Retrieved 2 May 2011.
- NCI Drug Dictionary: Glemtumumab vedotin Archived 5 April 2015 at the Wayback Machine
- "FDA approves Roche's Columvi, the first and only bispecific antibody with a fixed-duration treatment for people with relapsed or refractory diffuse large B-cell lymphoma" (Press release). F. Hoffmann-La Roche Ltd. 16 June 2023. Retrieved 16 June 2023 – via GlobeNewswire.
- "Results from Cure Brain Cancer Foundation-funded trial show promise". www.curebraincancer.org.au. Archived from the original on 19 March 2020. Retrieved 14 June 2019.
- "Uplizna- inebilizumab injection". DailyMed. 8 July 2019. Archived from the original on 13 June 2020. Retrieved 13 June 2020.
- Clinical trial number NCT00537381 for "A Study of the Safety and Effectiveness of CNTO 95 in Patients With Metastatic Hormone Refractory Prostate Cancer" at ClinicalTrials.gov
- Clinical trial number NCT00246012 for "A Study of the Safety and Efficacy CNTO 95 in Subjects With Advanced Melanoma" at ClinicalTrials.gov
- World Health Organization (2020). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 84". WHO Drug Information. 34 (3). hdl:10665/340680.
- "FDA Grants Accelerated Approval for Alzheimer's Disease Treatment". U.S. Food and Drug Administration (FDA) (Press release). 6 January 2023. Archived from the original on 7 January 2023. Retrieved 7 January 2023.
- World Health Organization (2018). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 79". WHO Drug Information. 32 (1). hdl:10665/330941.
- Guthrie, Arlo (27 June 2017). "Zoetis launches breakthrough treatment for canine atopic dermatitis". VetNurse News. Archived from the original on 7 July 2018. Retrieved 6 July 2018.
- "Margenza: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 23 March 2021. Retrieved 17 December 2020.
- "FDA grants accelerated approval to mirvetuximab soravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer". U.S. Food and Drug Administration (FDA). 14 November 2022. Archived from the original on 18 November 2022. Retrieved 18 November 2022.
- World Health Organization (1998). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 79" (PDF). WHO Drug Information. 12 (2). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- "FDA grants accelerated approval to mosunetuzumab-axgb". U.S. Food and Drug Administration. 22 December 2022. Archived from the original on 4 January 2023. Retrieved 3 January 2023.
- Wilde, Michelle I.; Goa, Karen L. (1 May 1996). "Muromonab CD3". Drugs. 51 (5): 865–894. doi:10.2165/00003495-199651050-00010. ISSN 1179-1950. PMID 8861551. S2CID 13220985.
- Hooks, M. A.; Wade, C. S.; Millikan, W. J. (1991). "Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation". Pharmacotherapy. 11 (1): 26–37. ISSN 0277-0008. PMID 1902291. Archived from the original on 10 September 2021. Retrieved 10 September 2021.
- World Health Organization (2006). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 96" (PDF). WHO Drug Information. 20 (4). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2008). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 100 (prepublication copy)" (PDF). WHO Drug Information. Archived (PDF) from the original on 29 August 2021. Retrieved 5 October 2020.
- Ruzicka, Thomas; Hanifin, Jon M.; Furue, Masutaka; Pulka, Grazyna; Mlynarczyk, Izabela; Wollenberg, Andreas; Galus, Ryszard; Etoh, Takafumi; Mihara, Ryosuke; Yoshida, Hiroki; Stewart, Jonathan; Kabashima, Kenji; XCIMA Study Group (2017). "Anti–Interleukin-31 Receptor a Antibody for Atopic Dermatitis". New England Journal of Medicine. 376 (9): 826–835. doi:10.1056/NEJMoa1606490. PMID 28249150. S2CID 205100502.
- World Health Organization (2006). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 56" (PDF). WHO Drug Information. 20 (3). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- World Health Organization (2006). "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 95" (PDF). WHO Drug Information. 20 (2). Archived (PDF) from the original on 15 July 2020. Retrieved 23 January 2020.
- "FDA approves Novartis Kesimpta (ofatumumab), the first and only self-administered, targeted B-cell therapy for patients with relapsing multiple sclerosis" (Press release). Novartis. 20 August 2020. Archived from the original on 24 September 2020. Retrieved 21 August 2020.
- "Keytruda- pembrolizumab injection, powder, lyophilized, for solution Keytruda- pembrolizumab injection, solution". DailyMed. 17 September 2019. Archived from the original on 17 June 2020. Retrieved 10 January 2020.
- "Regkirona EPAR". European Medicines Agency. 10 November 2021. Archived from the original on 12 November 2021. Retrieved 12 November 2021.
- "ZYNYZ™ (retifanlimab-dlwr) injection, for intravenous use" (PDF). Archived (PDF) from the original on 23 March 2023. Retrieved 23 March 2023.
- "Rituxan- rituximab injection, solution". DailyMed. 6 November 2019. Archived from the original on 4 August 2020. Retrieved 2 February 2020.
- "UCB announces U.S. FDA approval of Rystiggo (rozanolixizumab-noli) for the treatment of adults with generalized myasthenia gravis" (Press release). UCB. 27 June 2023. Retrieved 28 June 2023 – via PR Newswire.
- "Trodelvy- sacituzumab govitecan powder, for solution". DailyMed. Archived from the original on 20 March 2021. Retrieved 9 April 2021.
- Xie, B.; Shen, J.; Dong, A.; Rashid, A.; Stoller, G.; Campochiaro, P. A. (2009). "Blockade of Sphingosine-1-phosphate Reduces Macrophage Influx and Retinal and Choroidal Neovascularization". Journal of Cellular Physiology. 218 (1): 192–198. doi:10.1002/jcp.21588. PMC 2905312. PMID 18781584.
- "AusPAR: Sotrovimab". Therapeutic Goods Administration (TGA). 20 August 2021. Archived from the original on 1 November 2021. Retrieved 4 September 2021.
- "Xevudy EPAR". European Medicines Agency (EMA). 15 December 2021. Archived from the original on 18 December 2021. Retrieved 17 December 2021.
- World Health Organization (2019). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 81". WHO Drug Information. 33 (1). hdl:10665/330896.
- "Janssen Marks First Approval Worldwide for Tecvayli (teclistamab) with EC Authorisation of First-in-Class Bispecific Antibody for the Treatment of Patients with Multiple Myeloma". Janssen Pharmaceutical Companies (Press release). 24 August 2022. Archived from the original on 26 October 2022. Retrieved 26 October 2022.
- "U.S. FDA Approves Tecvayli (teclistamab-cqyv), the First Bispecific T-cell Engager Antibody for the Treatment of Patients with Relapsed or Refractory Multiple Myeloma". Janssen Pharmaceutical Companies. 25 October 2022. Retrieved 26 October 2022.
- Jostein Dahle; Ada H. V. Repetto-Llamazares; Camilla S. Mollatt; Katrine B. Melhus; Oyvind S. Bruland; Arne Kolstad; Roy H. Larsen (January 2013). "Evaluating antigen targeting and anti-tumor activity of a new anti-CD37 radioimmunoconjugate against non-Hodgkin's lymphoma". Anticancer Research. 33 (1): 85–95. PMID 23267131.
- "Tezspire- tezepelumab-ekko injection, solution". DailyMed. 17 December 2021. Archived from the original on 25 December 2021. Retrieved 28 June 2022.
- Wright, Rob (13 February 2018). "Seattle Genetics Legacy Of Partnering". Life Science Leader. Beyond The Printed Page. Archived from the original on 23 February 2018. Retrieved 22 February 2018.
- "Enforcement Reports" (PDF). Archived (PDF) from the original on 21 September 2021. Retrieved 21 September 2021.
- "RoActemra EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 18 December 2021. Retrieved 18 December 2021.
- World Health Organization (2010). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 64". WHO Drug Information. 24 (3). hdl:10665/74577.
- "LEO Pharma announces FDA approval of Adbry tralokinumab". LEO Pharma (Press release). Archived from the original on 28 December 2021. Retrieved 28 December 2021.
- Ruf, P; Jäger, M; Ellwart, J; Wosch, S; Kusterer, E; Lindhofer, H; et al. (2004). "Two new trifunctional antibodies for the therapy of human malignant melanoma". International Journal of Cancer. 108 (5): 725–732. doi:10.1002/ijc.11630. PMID 14696099. S2CID 25197165.
- World Health Organization (2008). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 59". WHO Drug Information. 22 (1). hdl:10665/74120.
- "Imjudo (tremelimumab) in combination with Imfinzi approved in the US for patients with unresectable liver cancer". AstraZeneca (Press release). 26 October 2022. Archived from the original on 24 October 2022. Retrieved 26 October 2022.
- World Health Organization (2011). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 66". WHO Drug Information. 25 (3). hdl:10665/74683.
- "Entyvio- vedolizumab injection, powder, lyophilized, for solution". DailyMed. 3 April 2020. Archived from the original on 21 October 2020. Retrieved 12 October 2020.
- "FDA authorizes Gohibic (vilobelimab) injection for the treatment of COVID-19". U.S. Food and Drug Administration (FDA). 4 April 2023. Retrieved 4 April 2023.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.