Neptunium(IV) fluoride
Neptunium(IV) fluoride or neptunium tetrafluoride is a inorganic compound with the formula NpF4. It is a green salt and is isostructural with UF4.[3]
 | |
| Names | |
|---|---|
| IUPAC name
Neptunium(IV) fluoride | |
| Other names
Neptunium tetrafluoride | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| NpF4 | |
| Molar mass | 313 g/mol |
| Appearance | Green solid[1] |
| Structure | |
| Monoclinic, mS60[1] | |
| C2/c, No. 15[2] | |
a = 1.27 nm, b = 1.0082 nm, c = 0.833 nm α = 90°, β = 126.03°, γ = 90° | |
Lattice volume (V) |
0.86256 nm3 |
Formula units (Z) |
12 |
| Thermochemistry | |
Heat capacity (C) |
116 ± 4 J/mol·K[1] |
Std molar entropy (S⦵298) |
148 ± 3 J/mol·K[1] |
Std enthalpy of formation (ΔfH⦵298) |
−1874 ± 16 kJ/mol[1] |
Gibbs free energy (ΔfG⦵) |
-1783 ± 16 kJ/mol[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
-fluorid.png.webp)
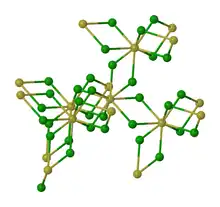
Alternative view of the structure of solid NpF4.
Neptunium(IV) fluoride can be prepared by reacting neptunium(III) fluoride or neptunium dioxide with a gas mixture of oxygen and hydrogen fluoride at 500 °C:[1]
It can also be prepared by treating neptunium dioxide with HF gas:[1]
References
- Haire, Richard G. (2006). "Neptunium". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. pp. 730–736. doi:10.1007/1-4020-3598-5_9. ISBN 1-4020-3555-1.
- Zachariasen, W. H. (1949). "Crystal chemical studies of the 5f-series of elements. XII. New compounds representing known structure types". Acta Crystallographica. 2 (6): 388–390. doi:10.1107/S0365110X49001016.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.