Nuclear receptor coactivator 3
The nuclear receptor coactivator 3 also known as NCOA3 is a protein that, in humans, is encoded by the NCOA3 gene.[5][6] NCOA3 is also frequently called 'amplified in breast 1' (AIB1), steroid receptor coactivator-3 (SRC-3), or thyroid hormone receptor activator molecule 1 (TRAM-1).
Function
NCOA3 is a transcriptional coactivator protein that contains several nuclear receptor interacting domains and an intrinsic histone acetyltransferase activity. NCOA3 is recruited to DNA promotion sites by ligand-activated nuclear receptors. NCOA3, in turn, acylates histones, which makes downstream DNA more accessible to transcription. Hence, NCOA3 assists nuclear receptors in the upregulation of gene expression.[7][8]
Clinical significance
The ratio of PAX2 to AIB-1 protein expression may be predictive of the effectiveness of tamoxifen in breast cancer treatment.[9][10]
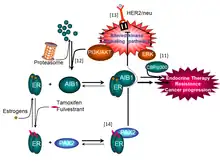
Several molecular mechanisms implicate NCOA3 (AIB1) in the endocrine therapy resistance (depicted in the figure). Signaling pathways or mutations (i.e. HER2/neu overexpression, activating mutations in PIK3CA (PI3K), activating mutations in the proto-oncogene tyrosine-protein kinase Src, etc.) that lead to persistent activation of ERK and/or PIK3CA/AKT kinase pathways result, in one hand in an enhanced AIB1 transcriptional coactivation capacity,[11] and in the other hand in the inhibition of the proteasome-dependent AIB1 turn-over and therefore, in AIB1 overexpression.[12] In both conditions, the equilibrium of estrogen receptor (ER) complex formation is displaced towards a transcriptionally active complex and thus, counteracting the inhibition caused by anti-estrogenic drugs such as tamoxifen or fulvestrant (selective estrogen receptor modulators). The result is the restoration of estrogen-sensitive gene transcription and the promotion of cancer progression and/or relapse.
Notably, tumors diagnosed with concomitant overexpression of AIB1 and HER2/neu have worse outcome with tamoxifen therapy than all other patients combined.[13] In addition, dormant tumor cells of luminal breast cancers treated with endocrine therapy may acquire with time, mutations that alter kinase signalling pathways and ultimately enhance AIB1 oncogenic functions. Also, estrogen receptor-PAX2 complexes repress HER2/neu expression, but loss of PAX2 expression may result in de novo HER2/neu expression and initiate endocrine therapy resistance and relapse.[14]
Interactions
Nuclear receptor coactivator 3 has been shown to interact with:
- Androgen receptor,[15][16][17]
- CHUK[18] and
- CREB-binding protein,[18][19]
- DDX17,[20]
- DDX5,[20]
- Estrogen receptor alpha,[20][21][22]
- Estrogen receptor beta,[21][23]
- Glucocorticoid receptor,[24][25]
- IKBKG,[18]
- IKK2,[18]
- Peroxisome proliferator-activated receptor gamma,[26] and
- Retinoid X receptor alpha.[27][28]
References
- GRCh38: Ensembl release 89: ENSG00000124151 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027678 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS (August 1997). "AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer". Science. 277 (5328): 965–8. doi:10.1126/science.277.5328.965. PMID 9252329.
- Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW (October 1997). "TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1". J. Biol. Chem. 272 (44): 27629–34. doi:10.1074/jbc.272.44.27629. PMID 9346901.
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS (1997). "AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer". Science. 277 (5328): 965–8. doi:10.1126/science.277.5328.965. PMID 9252329.
- Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW (1997). "TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1". J Biol Chem. 272 (44): 27629–34. doi:10.1074/jbc.272.44.27629. PMID 9346901.
- "Study sheds new light on tamoxifen resistance". Cordis News. Cordis. 2008-11-13. Archived from the original on 2009-02-20. Retrieved 2008-11-14.
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, et al. (December 2008). "Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen". Nature. 456 (7222): 663–6. Bibcode:2008Natur.456..663H. doi:10.1038/nature07483. PMC 2920208. PMID 19005469.
- Font de Mora J, Brown M (July 2000). "AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor". Molecular and Cellular Biology. 20 (14): 5041–7. doi:10.1128/MCB.20.14.5041-5047.2000. PMC 85954. PMID 10866661.
- Ferrero M, Avivar A, García-Macías MC, Font de Mora J (July 2008). "Phosphoinositide 3-kinase/AKT signaling can promote AIB1 stability independently of GSK3 phosphorylation". Cancer Research. 68 (13): 5450–9. doi:10.1158/0008-5472.CAN-07-6433. PMID 18593948.
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, et al. (March 2003). "Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer". Journal of the National Cancer Institute. 95 (5): 353–61. doi:10.1093/jnci/95.5.353. PMID 12618500.
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, et al. (December 2008). "Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen". Nature. 456 (7222): 663–6. Bibcode:2008Natur.456..663H. doi:10.1038/nature07483. PMC 2920208. PMID 19005469.
- Tan JA, Hall SH, Petrusz P, French FS (September 2000). "Thyroid receptor activator molecule, TRAM-1, is an androgen receptor coactivator". Endocrinology. 141 (9): 3440–50. doi:10.1210/endo.141.9.7680. PMID 10965917.
- Gnanapragasam VJ, Leung HY, Pulimood AS, Neal DE, Robson CN (December 2001). "Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer". Br. J. Cancer. 85 (12): 1928–36. doi:10.1054/bjoc.2001.2179. PMC 2364015. PMID 11747336.
- Wang Q, Udayakumar TS, Vasaitis TS, Brodie AM, Fondell JD (April 2004). "Mechanistic relationship between androgen receptor polyglutamine tract truncation and androgen-dependent transcriptional hyperactivity in prostate cancer cells". J. Biol. Chem. 279 (17): 17319–28. doi:10.1074/jbc.M400970200. PMID 14966121.
- Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, Tsai MJ, O'Malley BW (May 2002). "Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase". Mol. Cell. Biol. 22 (10): 3549–61. doi:10.1128/MCB.22.10.3549-3561.2002. PMC 133790. PMID 11971985.
- Naltner A, Wert S, Whitsett JA, Yan C (December 2000). "Temporal/spatial expression of nuclear receptor coactivators in the mouse lung". Am. J. Physiol. Lung Cell Mol. Physiol. 279 (6): L1066-74. doi:10.1152/ajplung.2000.279.6.l1066. PMID 11076796. S2CID 27872061.
- Watanabe M, Yanagisawa J, Kitagawa H, Takeyama K, Ogawa S, Arao Y, Suzawa M, Kobayashi Y, Yano T, Yoshikawa H, Masuhiro Y, Kato S (March 2001). "A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor alpha coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA". EMBO J. 20 (6): 1341–52. doi:10.1093/emboj/20.6.1341. PMC 145523. PMID 11250900.
- Wong CW, Komm B, Cheskis BJ (June 2001). "Structure-function evaluation of ER alpha and beta interplay with SRC family coactivators. ER selective ligands". Biochemistry. 40 (23): 6756–65. doi:10.1021/bi010379h. PMID 11389589.
- Tikkanen MK, Carter DJ, Harris AM, Le HM, Azorsa DO, Meltzer PS, Murdoch FE (November 2000). "Endogenously expressed estrogen receptor and coactivator AIB1 interact in MCF-7 human breast cancer cells". Proc. Natl. Acad. Sci. U.S.A. 97 (23): 12536–40. Bibcode:2000PNAS...9712536T. doi:10.1073/pnas.220427297. PMC 18799. PMID 11050174.
- Leo C, Li H, Chen JD (February 2000). "Differential mechanisms of nuclear receptor regulation by receptor-associated coactivator 3". J. Biol. Chem. 275 (8): 5976–82. doi:10.1074/jbc.275.8.5976. PMID 10681591.
- Hsiao PW, Fryer CJ, Trotter KW, Wang W, Archer TK (September 2003). "BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation". Mol. Cell. Biol. 23 (17): 6210–20. doi:10.1128/MCB.23.17.6210-6220.2003. PMC 180928. PMID 12917342.
- Zilliacus J, Holter E, Wakui H, Tazawa H, Treuter E, Gustafsson JA (April 2001). "Regulation of glucocorticoid receptor activity by 14--3-3-dependent intracellular relocalization of the corepressor RIP140". Mol. Endocrinol. 15 (4): 501–11. doi:10.1210/mend.15.4.0624. PMID 11266503.
- Kodera Y, Takeyama K, Murayama A, Suzawa M, Masuhiro Y, Kato S (October 2000). "Ligand type-specific interactions of peroxisome proliferator-activated receptor gamma with transcriptional coactivators". J. Biol. Chem. 275 (43): 33201–4. doi:10.1074/jbc.C000517200. PMID 10944516.
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM (August 1997). "Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300". Cell. 90 (3): 569–80. doi:10.1016/S0092-8674(00)80516-4. PMID 9267036. S2CID 15284825.
- Lee WY, Noy N (February 2002). "Interactions of RXR with coactivators are differentially mediated by helix 11 of the receptor's ligand binding domain". Biochemistry. 41 (8): 2500–8. doi:10.1021/bi011764+. PMID 11851396.
External links
- nuclear receptor coactivator 3 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- NURSA C91
Further reading
- Liao L, Kuang SQ, Yuan Y, Gonzalez SM, O'Malley BW, Xu J (2003). "Molecular structure and biological function of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1". J. Steroid Biochem. Mol. Biol. 83 (1–5): 3–14. doi:10.1016/S0960-0760(02)00254-6. PMID 12650696. S2CID 40981759.
- Liu F, Ventura F, Doody J, Massagué J (1995). "Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs". Mol. Cell. Biol. 15 (7): 3479–86. doi:10.1128/mcb.15.7.3479. PMC 230584. PMID 7791754.
- Guan XY, Xu J, Anzick SL, Zhang H, Trent JM, Meltzer PS (1996). "Hybrid selection of transcribed sequences from microdissected DNA: isolation of genes within amplified region at 20q11-q13.2 in breast cancer". Cancer Res. 56 (15): 3446–50. PMID 8758910.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Margolis RL, Abraham MR, Gatchell SB, Li SH, Kidwai AS, Breschel TS, Stine OC, Callahan C, McInnis MG, Ross CA (1997). "cDNAs with long CAG trinucleotide repeats from human brain". Hum. Genet. 100 (1): 114–22. doi:10.1007/s004390050476. PMID 9225980. S2CID 25999127.
- Li H, Gomes PJ, Chen JD (1997). "RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2". Proc. Natl. Acad. Sci. U.S.A. 94 (16): 8479–84. Bibcode:1997PNAS...94.8479L. doi:10.1073/pnas.94.16.8479. PMC 22964. PMID 9238002.
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS (1997). "AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer". Science. 277 (5328): 965–8. doi:10.1126/science.277.5328.965. PMID 9252329.
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM (1997). "Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300". Cell. 90 (3): 569–80. doi:10.1016/S0092-8674(00)80516-4. PMID 9267036. S2CID 15284825.
- Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW (1997). "TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1". J. Biol. Chem. 272 (44): 27629–34. doi:10.1074/jbc.272.44.27629. PMID 9346901.
- Korzus E, Torchia J, Rose DW, Xu L, Kurokawa R, McInerney EM, Mullen TM, Glass CK, Rosenfeld MG (1998). "Transcription factor-specific requirements for coactivators and their acetyltransferase functions". Science. 279 (5351): 703–7. Bibcode:1998Sci...279..703K. doi:10.1126/science.279.5351.703. PMID 9445475.
- Shirazi SK, Bober MA, Coetzee GA (1998). "Polymorphic exonic CAG microsatellites in the gene amplified in breast cancer (AIB1 gene)". Clin. Genet. 54 (1): 102–3. doi:10.1111/j.1399-0004.1998.tb03704.x. PMID 9727751. S2CID 1315265.
- Wang JC, Stafford JM, Granner DK (1998). "SRC-1 and GRIP1 coactivate transcription with hepatocyte nuclear factor 4". J. Biol. Chem. 273 (47): 30847–50. doi:10.1074/jbc.273.47.30847. PMC 3968904. PMID 9812974.
- Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R (1999). "Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1". Genes Dev. 12 (22): 3488–98. doi:10.1101/gad.12.22.3488. PMC 317237. PMID 9832502.
- Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM (1999). "Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase". Cell. 98 (5): 675–86. doi:10.1016/S0092-8674(00)80054-9. PMID 10490106. S2CID 14697597.
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T (2000). "Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation". J. Cell Sci. 112 (20): 3519–27. doi:10.1242/jcs.112.20.3519. PMID 10504300.
- Xie W, Hong H, Yang NN, Lin RJ, Simon CM, Stallcup MR, Evans RM (2000). "Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2". Mol. Endocrinol. 13 (12): 2151–62. doi:10.1210/mend.13.12.0381. PMID 10598588.
- Pao GM, Janknecht R, Ruffner H, Hunter T, Verma IM (2000). "CBP/p300 interact with and function as transcriptional coactivators of BRCA1". Proc. Natl. Acad. Sci. U.S.A. 97 (3): 1020–5. Bibcode:2000PNAS...97.1020P. doi:10.1073/pnas.97.3.1020. PMC 15508. PMID 10655477.
- Leo C, Li H, Chen JD (2000). "Differential mechanisms of nuclear receptor regulation by receptor-associated coactivator 3". J. Biol. Chem. 275 (8): 5976–82. doi:10.1074/jbc.275.8.5976. PMID 10681591.
- Font de Mora J, Brown M (2000). "AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor". Mol. Cell. Biol. 20 (14): 5041–7. doi:10.1128/MCB.20.14.5041-5047.2000. PMC 85954. PMID 10866661.
- Tan JA, Hall SH, Petrusz P, French FS (2000). "Thyroid receptor activator molecule, TRAM-1, is an androgen receptor coactivator". Endocrinology. 141 (9): 3440–50. doi:10.1210/endo.141.9.7680. PMID 10965917.