Cetyl alcohol
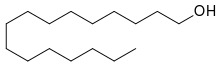
Cetyl alcohol /ˈsiːtəl/, also known as hexadecan-1-ol and palmityl alcohol, is a C-16 fatty alcohol with the formula CH3(CH2)15OH. At room temperature, cetyl alcohol takes the form of a waxy white solid or flakes. The name cetyl derives from the whale oil (cetacea oil, from Latin: cetus, lit. 'whale', from Ancient Greek: κῆτος, romanized: kētos, lit. 'huge fish')[3] from which it was first isolated.[4]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hexadecan-1-ol | |
| Other names
Cetanol, Cetyl alcohol, Ethal, Ethol, Hexadecanol, Hexadecyl alcohol, Palmityl alcohol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.048.301 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H34O | |
| Molar mass | 242.447 g·mol−1 |
| Appearance | White crystals or flakes |
| Odor | Very faint, waxy |
| Density | 0.811 g/cm3 |
| Melting point | 49.3 °C (120.7 °F; 322.4 K) |
| Boiling point | 344 °C (651 °F; 617 K) |
| Insoluble | |
| Solubility | Very soluble in ether, benzene, and chloroform. Soluble in acetone. Slightly soluble in alcohol. |
| log P | 7.25[2] |
| Acidity (pKa) | 16.20 |
| −183.5·10−6 cm3/mol | |
Refractive index (nD) |
1.4283 (79 °C) |
| Viscosity | 53 cP (75 °C) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 185 °C (365 °F; 458 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
5000 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
Cetyl alcohol was discovered in 1817 by the French chemist Michel Chevreul when he heated spermaceti, a waxy substance obtained from sperm whale oil, with caustic potash (potassium hydroxide). Flakes of cetyl alcohol were left behind on cooling.[5] Modern production is based around the chemical reduction of ethyl palmitate.[6]
Uses
Cetyl alcohol is used in the cosmetic industry as an opacifier in shampoos, or as an emollient, emulsifier or thickening agent in the manufacture of skin creams and lotions.[7] It is also employed as a lubricant for nuts and bolts, and is the active ingredient in some "liquid pool covers" (forming a non-volatile surface layer to reduce water evaporation, related latent vaporization heat loss, and thus to retain heat in the pool). Moreover, it can also be used as a non-ionic co-surfactant in emulsion applications.[8]
Side effects
People who suffer from eczema can be sensitive to cetyl alcohol,[9][10] though this may be due to impurities rather than cetyl alcohol itself.[11] However, cetyl alcohol is sometimes included in medications used for the treatment of eczema.[12]
Related compounds
References
- Merck Index, 11th Edition, 2020.
- "Hexadecan-1-ol_msds".
- M. Raneft, D.; Eaker, H.; W. Davis, R. (2001). "A guide to the pronunciation and meaning of cetacean taxonomic names" (PDF). Aquatic Mammals. 27 (2): 185.
- Nordegren, Thomas (2002). The A-Z Encyclopedia of Alcohol and Drug Abuse. Universal Publishers. p. 165. ISBN 1-58112-404-X.
- Booth, James Curtis (1862). The Encyclopedia of Chemistry, Practical and Theoretical. Philadelphia, H.C. Baird. p. 429.
- "Cetyl alcohol". Encyclopedia Britannica. July 20, 1998. Retrieved 2023-01-28.
- Smolinske, Susan C (1992). Handbook of Food, Drug, and Cosmetic Excipients. CRC Press. pp. 75–76. ISBN 0-8493-3585-X.
- Golemanov, Konstantin; Tcholakova, Slavka; Denkov, Nikolai D.; Gurkov, Theodor (April 2006). "Selection of surfactants for stable paraffin-in-water dispersions, undergoing solid−liquid transition of the dispersed particles". Langmuir. 22 (8): 3560–3569. doi:10.1021/la053059y. ISSN 0743-7463. PMID 16584227.
- Gaul, LE (1969). "Dermatitis from cetyl and stearyl alcohols". Archives of Dermatology. 99 (5): 593. doi:10.1001/archderm.1969.01610230085016. PMID 4238421.
- Soga, F; Katoh, N; Kishimoto, S (2004). "Contact dermatitis due to lanoconazole, cetyl alcohol and diethyl sebacate in lanoconazole cream". Contact Dermatitis. 50 (1): 49–50. doi:10.1111/j.0105-1873.2004.00271j.x. PMID 15059111. S2CID 19854024.
- Komamura, H; Doi, T; Inui, S; Yoshikawa, K (1997). "A case of contact dermatitis due to impurities of cetyl alcohol". Contact Dermatitis. 36 (1): 44–6. doi:10.1111/j.1600-0536.1997.tb00921.x. PMID 9034687. S2CID 23444831.
- Kato N; Numata T; Kanzaki T (1987). "Contact dermatitis due to Japanese pharmacopeia cetyl alcohol". Skin Research. 29 (suppl 3): 258–262.
