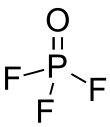
Phosphoryl fluoride
Phosphoryl fluoride (commonly called phosphorus oxyfluoride) is a compound with the chemical formula POF3. It is a colorless gas that hydrolyzes rapidly. It has a critical temperature of 73 °C and a critical pressure of 4.25 bars.[1]
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Phosphoryl trifluoride Phosphorus trifluoride oxide | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.033.419 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| POF3 | |||
| Molar mass | 103.9684 g/mol | ||
| Appearance | Colourless gas | ||
| Boiling point | −39.7 °C (−39.5 °F; 233.5 K) | ||
| Reacts | |||
| Solubility | Reacts with alcohol and acid, soluble in diethyl ether and hydrocarbons | ||
| 1.76 D[1] | |||
| Structure | |||
| Tetrahedral at the P atom | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Poison, corrosive, can form HF on contact with H2O | ||
| GHS labelling: | |||
    | |||
| Danger | |||
| H302, H314, H330, H372 | |||
| P260, P264, P270, P271, P280, P284, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P314, P320, P321, P330, P363, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | ICSC 0190 | ||
| Related compounds | |||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Synthesis and reactions
Phosphorus oxyfluoride is prepared by partial hydrolysis of phosphorus pentafluoride.
Phosphorus oxyfluoride is the progenitor of the simple fluorophosphoric acids by hydrolysis. The sequence starts with difluorophosphoric acid:
- POF3 + H2O → HPO2F2 + HF
The next steps give monofluorophosphoric acid and phosphoric acid:
- HPO2F2 + H2O → H2PO3F + HF
- HPO3F + H2O → H3PO4 + 5 HF
Phosphoryl fluoride combines with dimethylamine to produce dimethylaminophosphoryl difluoride (H3C−)2N−P(=O)F2 and difluorophosphate and hexafluorophosphate ions.[2]
References
- https://www.stenutz.eu/chem/solv6.php?name=phosphoryl+fluoride
- Cavell, R. G. (1968). "Chemistry of phosphorus fluorides. Part III. The reaction of thiophosphoryl-fluoride with dimethylamine and some properties of the dimethylaminothio- phosphoryl fluorides". Canadian Journal of Chemistry. 46 (4): 613. doi:10.1139/v68-100.
Wikimedia Commons has media related to Phosphoryl fluoride.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.