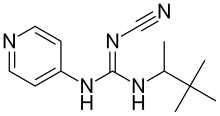
Pinacidil
Pinacidil is a cyanoguanidine drug that opens ATP-sensitive potassium channels producing peripheral vasodilatation of arterioles.[1] It reduces blood pressure and peripheral resistance and produces fluid retention.[2]
 | |
| Names | |
|---|---|
| IUPAC name
N-cyano-N'-pyridin-4-yl-N''-(1,2,2-trimethylpropyl)guanidine | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.056.614 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H19N5 | |
| Molar mass | 245.32346 |
| Pharmacology | |
| C02DG01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
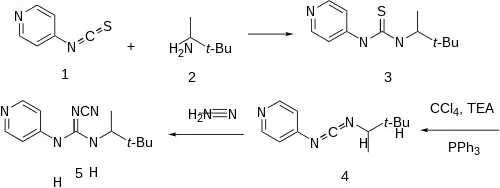
Condensation of 4-isothiocyanotopyridine [76105-84-5] (1) and 3,3-dimethyl-2-butanamine [3850-30-4] (2) gives thiourea [67027-06-9] (3). Treatment of that intermediate with a mixture of triphenylphosphine, carbon tetrachloride, and triethylamine leads to the unsymmetrical carbodiimide, CID:20501933 (4'). Addition of cyanamid affords pinacidil (5).
References
- Gollasch M, Bychkov R, Ried C, Behrendt F, Scholze S, Luft FC, Haller H (1995). "Pinacidil relaxes porcine and human coronary arteries by activating ATP-dependent potassium channels in smooth muscle cells". J. Pharmacol. Exp. Ther. 275 (2): 681–92. PMID 7473155.
- Reynolds, James Blair; Martindale, William L. (1996). The extra pharmacopoeia (31st ed.). London: Royal Pharmaceutical Society. pp. 2739 pages. ISBN 0-85369-342-0.
- Petersen, Hans Joergen; Nielsen, C. Kaergaard; Arrigoni-Martelli, E. (1978). "Synthesis and hypotensive activity of N-alkyl-N-cyano-N'-pyridylguanidines". Journal of Medicinal Chemistry 21 (8): 773–781. doi:10.1021/jm00206a011.
- Hansen, E. T.; Petersen, H. J. (2006). "Synthesis ofN-Alkyl-N'-cyano-N″-4-pyridylguanidines from 4-Pyridyldithiocarbamic AcidviaN-Alkyl-N′-4-Pyridylthioureas, orvia4-Pyridylcyaniminothiocarbamic Acid". Synthetic Communications. 14 (13): 1275–1283. doi:10.1080/00397918408076809.
- Zhang, Hao; Liu, Rui-Quan; Liu, Ke-Chang; Li, Qi-Bo; Li, Qing-Yang; Liu, Shang-Zhong (2014). "A One-Pot Approach to Pyridyl Isothiocyanates from Amines". Molecules 19(9): 13631–13642. doi:10.3390/molecules190913631.
- Hans J. Petersen, USRE31244E (1983 to Leo Pharma AS).
- Hans Jorgen Petersen, U.S. Patent 4,057,636 (1977 to Leo Pharma AS).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.