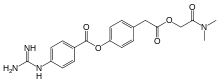
Camostat
Camostat is a serine protease inhibitor. Serine protease enzymes have a variety of functions in the body, and so camostat has a diverse range of uses. Camostat is approved in Japan for the treatment of chronic pancreatitis and postoperative reflux esophagitis.[1][2] The oral proteolytic enzyme inhibitor has been on the market since 1985 under the trade name Foipan Tablets. The manufacturer is Ono Pharmaceutical. The drug is used in the treatment of some forms of cancer and is also effective against some viral infections, as well as inhibiting fibrosis in liver or kidney disease or pancreatitis.[3][4][5][6][7]
 | |
| Clinical data | |
|---|---|
| Trade names | Foipan |
| Other names | FOY-305 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H22N4O5 |
| Molar mass | 398.419 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Pharmacology
It is an inhibitor of the enzyme transmembrane protease, serine 2 (TMPRSS2). For chronic pancreatitis camostat's typical dose is 600 mg daily, for postoperative reflux esophagitis 300 mg are taken. The daily dose is split in 3 doses and taken after each meal.[1]
Side effects
As side effects allergic reactions including anaphylaxis, hypersensitivity, hyperkalemia, platelet and leukocyte depletion, liver dysfunction, jaundice have been reported.[8]
COVID-19
Inhibition of TMPRSS2 partially blocked infection by SARS-CoV and Human coronavirus NL63 in HeLa cell cultures.[9] Another in vitro study showed that camostat significantly reduces the infection of Calu-3 lung cells by SARS-CoV-2, the virus responsible for COVID-19.[10][11] It is currently in many Phase 1 and Phase 2 clinical trials.[12][13][14]
Camostat decreased CRP levels better compared to Lopinavir/Ritonavir in a small study of mild COVID-19 patients.[15] Camostat decreased COVID-19 severity, improved inflammatory markers and oxygenation compared to hydroxychloroquine treated patients.[16][11]
A study of 205 COVID-19 patients treated with Camostat, carried out at Aarhus University Hospital in Denmark and concluding in April 2021, showed no noticeable effects of Camostat on duration of hospitalisation or severity of the cases, but noted that higher doses (the study used 600 mg Camostat daily dosage) might still have a possible effect.[17]
On July 1, 2021, the AIDS Clinical Trials Group announced that the Camostat group on the "ACTIV-2 Outpatient Monoclonal Antibodies and Other Therapies Trial" would not be moving forward to Phase 3. The trial demonstrated no safety concerns but also no changes in viral shedding or symptom improvement.[18]
References
- http://www.shijiebiaopin.net/upload/product/201272318373223.PDF
- "Camostat". drugs.com.
- Okuno, M.; Kojima, S.; Akita, K.; Matsushima-Nishiwaki, R.; Adachi, S.; Sano, T.; Takano, Y.; Takai, K.; Obora, A.; Yasuda, I.; Shiratori, Y.; Okano, Y.; Shimada, J.; Suzuki, Y.; Muto, Y.; Moriwaki, Y. (2002). "Retinoids in liver fibrosis and cancer". Frontiers in Bioscience. 7 (4): d204-18. doi:10.2741/A775. PMID 11779708.
- Hsieh, H. P.; Hsu, J. T. (2007). "Strategies of development of antiviral agents directed against influenza virus replication". Current Pharmaceutical Design. 13 (34): 3531–42. doi:10.2174/138161207782794248. PMID 18220789.
- Kitamura, K.; Tomita, K. (2012). "Proteolytic activation of the epithelial sodium channel and therapeutic application of a serine protease inhibitor for the treatment of salt-sensitive hypertension". Clinical and Experimental Nephrology. 16 (1): 44–8. doi:10.1007/s10157-011-0506-1. PMID 22038264. S2CID 6522071.
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion Jr, R.; Nunneley, J. W.; Barnard, D.; Pöhlmann, S.; McKerrow, J. H.; Renslo, A. R.; Simmons, G. (2015). "Protease inhibitors targeting coronavirus and filovirus entry". Antiviral Research. 116: 76–84. doi:10.1016/j.antiviral.2015.01.011. PMC 4774534. PMID 25666761.
- Ueda, M.; Uchimura, K.; Narita, Y.; Miyasato, Y.; Mizumoto, T.; Morinaga, J.; Hayata, M.; Kakizoe, Y.; Adachi, M.; Miyoshi, T.; Shiraishi, N.; Kadowaki, D.; Sakai, Y.; Mukoyama, M.; Kitamura, K. (2015). "The serine protease inhibitor camostat mesilate attenuates the progression of chronic kidney disease through its antioxidant effects". Nephron. 129 (3): 223–32. doi:10.1159/000375308. PMID 25766432. S2CID 207652863.
- "医療用医薬品 : カモスタットメシル酸塩 (カモスタットメシル酸塩錠100mg「日医工」)". KEGG (in Japanese). Retrieved 2021-02-05.
- Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S (June 2012). "Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry". J. Virol. 86 (12): 6537–45. doi:10.1128/JVI.00094-12. PMC 3393535. PMID 22496216.
- Hoffman, Markus (2020-03-05). "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor". Cell. 181 (2): 271–280.e8. doi:10.1016/j.cell.2020.02.052. PMC 7102627. PMID 32142651.
- Jackson CB, Farzan M, Chen B, Choe H (2022). "Mechanisms of SARS-CoV-2 entry into cells". Nature Reviews Molecular Cell Biology. 23 (1): 3–20. doi:10.1038/s41580-021-00418-x. PMC 8491763. PMID 34611326.
- "Novel Agents for Treatment of High-risk COVID-19 Positive Patients - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2020-06-12.
- "The Impact of Camostat Mesilate on COVID-19 Infection - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2020-06-12.
- "Search of: camostat - covid - List Results". ClinicalTrials.gov. Retrieved 2021-02-04.
- Choi, Jae-Phil; Kim, Hyoung-Jun; Han, Jumi; Park, Sujung; Han, JinJoo (2020). "Foistar®(Camostat mesylate) associated with the significant decrease in CRP levels compared to Kaletra®(Lopinavir/Ritonavir) treatment in Korean mild COVID-19 pneumonic patients". medRxiv. doi:10.1101/2020.12.10.20240689. S2CID 229180615.
{{cite journal}}: Missing|author2=(help) - Hofmann-Winkler H, Moerer O, Alt-Epping S, Bräuer A, Büttner B, Müller M; et al. (2020). "Camostat Mesylate May Reduce Severity of Coronavirus Disease 2019 Sepsis: A First Observation". Crit Care Explor. 2 (11): e0284. doi:10.1097/CCE.0000000000000284. hdl:20.500.11850/460814. PMC 7671878. PMID 33225308.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "Middel mod halsbrand bremsede ikke Covid-19". newsroom.au.dk (in Danish). Retrieved 2021-04-28.
- "ACTG announces Camostat will not advance to phase 3 in outpatient treatment study for COVID-19". EurekAlert!. Retrieved 2021-07-01.
External links
- Kunze H, Bohn E (May 1983). "Effects of the serine protease inhibitors FOY and FOY 305 on phospholipase A1 (EC 3.1.1.32) activity in rat - liver lysosomes". Pharmacol Res Commun. 15 (5): 451–9. doi:10.1016/S0031-6989(83)80065-4. PMID 6412250.
- Göke B, Stöckmann F, Müller R, Lankisch PG, Creutzfeldt W (1984). "Effect of a specific serine protease inhibitor on the rat pancreas: systemic administration of camostate and exocrine pancreatic secretion". Digestion. 30 (3): 171–8. doi:10.1159/000199102. PMID 6209186.