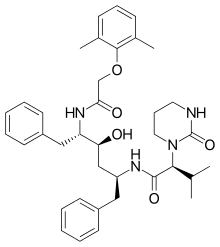
Lopinavir
Lopinavir is an antiretroviral of the protease inhibitor class. It is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir (lopinavir/ritonavir).[1]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | ABT-378 |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a602015 |
| License data | |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 98-99% |
| Metabolism | Liver |
| Elimination half-life | 5 to 6 hours |
| Excretion | Mostly fecal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C37H48N4O5 |
| Molar mass | 628.814 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
It was patented in 1995 and approved for medical use in 2000.[2]
Side effects
Side effects, interactions, and contraindications have only been evaluated in the drug combination lopinavir/ritonavir.
Pharmacology
Lopinavir is highly bound to plasma proteins (98–99%).[3]
Reports are contradictory regarding lopinavir penetration into the cerebrospinal fluid (CSF). Anecdotal reports state that lopinavir cannot be detected in the CSF; however, a study of paired CSF-plasma samples from 26 patients receiving lopinavir/ritonavir found lopinavir CSF levels above the IC50 in 77% of samples.[4]
Research
A 2014 study indicates that lopinavir is effective against the human papilloma virus (HPV). The study used the equivalent of one tablet twice a day applied topically to the cervices of women with high-grade and low-grade precancerous conditions. After three months of treatment, 82.6% of the women who had high-grade disease had normal cervical conditions, confirmed by smears and biopsies.[5] Lopinavir has been shown to impair protein synthesis via AMP-activated protein kinase (AMPK) and eEF2 kinase (eEF2K) activation, a mechanism that is similar to the antiviral effect of protein phosphatase 1 inhibitors.[6][7]
Lopinavir was found to inhibit MERS-CoV replication in the low-micromolar range in cell cultures.[8] In 2020, lopinavir/ritonavir was found not to work in severe COVID-19. In this trial the medication was started typically around 13 days after the start of symptoms.[9]
References
- "FDA Approved Drug Products: Kaletra". Retrieved 30 April 2004.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 510. ISBN 9783527607495.
- Kaletra (lopinavir/ritonavir) capsules; (lopinavir/ritonavir) oral solution. Prescribing information. April 2009
- Capparelli EV, Holland D, Okamoto C, Gragg B, Durelle J, Marquie-Beck J, et al. (June 2005). "Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV". AIDS. 19 (9): 949–52. doi:10.1097/01.aids.0000171409.38490.48. PMID 15905676. S2CID 3162858.
- HIV drug used to reverse effects of virus that causes cervical cancer University of Manchester, 17 February 2014.
- Stecher C, Marinkov S, Mayr-Harting L, Katic A, Kastner MT, Rieder-Rommer FJ, et al. (2021). "Protein phosphatase 1 regulates Human Cytomegalovirus protein translation by restraining AMPK signaling". Frontiers in Microbiology. 12: 698603. doi:10.3389/fmicb.2021.698603. ISSN 1664-302X. PMC 8320725. PMID 34335531.
- Ammosova T, Platonov M, Ivanov A, Kont YS, Kumari N, Kehn-Hall K, et al. (November 2014). "1E7-03, a low MW compound targeting host protein phosphatase-1, inhibits HIV-1 transcription". British Journal of Pharmacology. 171 (22): 5059–75. doi:10.1111/bph.12863. PMC 4253456. PMID 25073485.
- de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, et al. (August 2014). "Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture". Antimicrobial Agents and Chemotherapy. 58 (8): 4875–84. doi:10.1128/AAC.03011-14. PMC 4136071. PMID 24841269.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. (May 2020). "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19". The New England Journal of Medicine. 382 (19): 1787–1799. doi:10.1056/NEJMoa2001282. PMC 7121492. PMID 32187464.
External links
- "Lopinavir". Drug Information Portal. U.S. National Library of Medicine.