Dexelvucitabine
Dexelvucitabine is a failed experimental agent for the management of human immunodeficiency virus infection. It is a cytidine nucleoside analog and nucleoside reverse transcriptase inhibitor.[1] that inhibits HIV-1 replication in vitro. During phase II clinical trials there was some indication of a decreased mean viral load in patients with infected human immunodeficiency virus.[2][3]
 | |
| Names | |
|---|---|
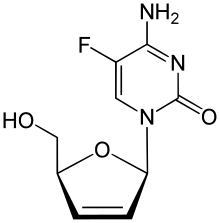
| Preferred IUPAC name
4-Amino-5-fluoro-1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-1-yl]pyrimidin-2(1H)-one | |
| Other names
Reverset | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C9H10FN3O3 |
| Molar mass | 227.195 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
On April 3, 2006, Pharmasset and Incyte, the pharmaceutical companies developing dexelvucitabine, announced the decision to cease further trials and development of the drug due to an increased incidence of grade 4 hyperlipasemia (an excess of the pancreatic enzyme lipase in the bloodstream) in a phase II trial.[1][4]
References
- PubChem. "Dexelvucitabine". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-04-07.
- Hernandez-Santiago, Brenda I.; Mathew, Judy S.; Rapp, Kim L.; Grier, Jason P.; Schinazi, Raymond F. (June 2007). "Antiviral and Cellular Metabolism Interactions between Dexelvucitabine and Lamivudine". Antimicrobial Agents and Chemotherapy. 51 (6): 2130–2135. doi:10.1128/aac.01543-06. ISSN 0066-4804. PMC 1891415. PMID 17403996.
- Sobieszczyk, Magdalena E; Talley, Angela K; Wilkin, Timothy; Hammer, Scott M (2005-03-01). "Advances in antiretroviral therapy". Topics in HIV Medicine. 13 (1): 24–44. ISSN 2161-5845. PMID 15849370.
- Ryder, Neil S (2007-12-01). "Discontinued drugs in 2006: anti-infectives". Expert Opinion on Investigational Drugs. 16 (12): 1867–1878. doi:10.1517/13543784.16.12.1867. ISSN 1354-3784. PMID 18041997. S2CID 40129603.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.