Seliciclib
Seliciclib (roscovitine or CYC202) is an experimental drug candidate in the family of pharmacological cyclin-dependent kinase (CDK) inhibitors that preferentially inhibit multiple enzyme targets including CDK2, CDK7 and CDK9, which alter the growth phase or state within the cell cycle of treated cells. Seliciclib is being developed by Cyclacel.This is a phase II, dose ranging, multicenter, randomized, double-blind, placebo-controlled study.
 | |
| Names | |
|---|---|
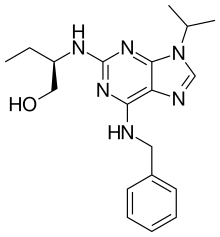
| Preferred IUPAC name
(2R)-2-{[6-(Benzylamino)-9-(propan-2-yl)-9H-purin-2-yl]amino}butan-1-ol | |
| Other names
Roscovitine; CYC202 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| MeSH | roscovitine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C19H26N6O |
| Molar mass | 354.458 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The aim of this study is to assess the safety of increasing doses of roscovitine administered orally for 4 cycles of 4 consecutive days (treatment "on") separated by a 3 days treatment free period (treatment "off") in adult CF subjects with Cystic Fibrosis carrying 2 Cystic Fibrosis causing mutations with at least one F508del-CFTR mutation and chronically infected with Pseudomonas aeruginosa.
This study involved 36 Cystic Fibrosis patients: 24 treated and 12 controls.[1]
Seliciclib is being researched for the treatment of non-small cell lung cancer (NSCLC), Cushing's disease, leukemia, HIV infection, Parkinson’s disease, herpes simplex infection, cystic fibrosis[2] and the mechanisms of chronic inflammation disorders.
Seliciclib is a 2,6,9-substituted purine analog. Its structure in complex with CDK2 was determined in 1996.[3] Seliciclib inhibits CDK2/E, CDK2/A, CDK7 and CDK9.[4]
Uses
Seliciclib has been found to produce apoptosis in treated cancerous cells of non-small cell lung cancer (NSCLC) and other cancers. Seliciclib has previously undergone Phase IIa clinical trials, in 240 NSCLC patients as a combined dose with existing first- and second-line treatments.[4][5] In the current APPRAISE trial, the research drug is undergoing Phase IIb clinical trial as a monotherapy for NSCLC in third-line patients.[6] The side-effects reported in Phase I trials of seliciclib for NSCLC were "nausea, vomiting, transient elevations in serum creatinine and liver function parameters and transient hypokalemia".[5]
Seliciclib is also in clinical trials for B-cell lymphomas, including multiple myeloma. Seliciclib has been shown to inhibit RNA polymerase II-dependent transcription and down-regulation of the protein MCL1.[7][8]
In the nervous system, seliciclib has been shown to suppress microglial activation[9] and to provide some neuroprotection in animal models of cerebral ischemia.[10][11] Furthermore, it increases antitumor activity of temozolomide in treatment of glioblastoma multiforme and is considered as a possible therapeutic option for glioma.[12]
Seliciclib is also a possible antiviral agent. It causes the death of cells infected with HIV[13][14][15] and preventing the replication of herpes simplex virus.[16][17]
Seliciclib has been shown in vitro to induce apoptosis in neutrophil granulocytes.[18] If this mechanism turns out to be safe, reliable and efficient in vivo, the drug could improve treatment of chronic inflammation diseases such as cystic fibrosis and arthritis. These are usually treated with glucocorticoids which often have serious side effects.
Seliciclib has been shown to cause parthogenetic egg activation. However it does create abnormal second polar bodies and therefore possible aneuploid zygotes. Egg activation usually involves calcium oscillations however this does not happen with seliciclib. Seciclib causes egg activation by inhibiting protein kinases which results in the inactivation of the maturation promoting factor (MPF).[19]
References
- "A Phase II, Dose Ranging, Multicenter, Double-blind, Placebo Controlled Study to Evaluate Safety and Effects of (R)-Roscovitine in Adults Subjects with Cystic Fibrosis, Carrying 2 Cystic Fibrosis Causing Mutations with at Least One F508del-CFTR Mutation and Chronically Infected with Pseudomonas Aeruginosa, a Study Involving 36 CF Patients (24 Treated, 12 Controls). ROSCO-CF". 11 December 2018.
{{cite journal}}: Cite journal requires|journal=(help) - Noel S, Faveau C, Norez C, Rogier C, Mettey Y, Becq F (2006). "Discovery of pyrrolo[2,3-b]pyrazines derivatives as submicromolar affinity activators of wild type, G551D, and F508del cystic fibrosis transmembrane conductance regulator chloride channels". J Pharmacol Exp Ther. 319 (1): 349–59. doi:10.1124/jpet.106.104521. PMID 16829626. S2CID 1554921.
- De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH (1997). "Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine". Eur J Biochem. 243 (1–2): 518–526. doi:10.1111/j.1432-1033.1997.0518a.x. PMID 9030780.
- "Cyclacel Begins a Phase IIb Randomized Trial of Seliciclib for Previously Treated Non-Small Cell Lung Cancer". BIOWIRE. June 29, 2006.
- "Cyclacel Reports Interim Seliciclib Phase IIa Data at 2005 ASCO". Business Wire. May 15, 2005.
- "Cyclacel Pharmaceuticals Reports Second Quarter 2006 Financial Results". Business Wire. August 14, 2006.
- MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A, Lane DP, Green SR (2005). "Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1". Cancer Research. 65 (12): 5399–5407. doi:10.1158/0008-5472.CAN-05-0233. PMID 15958589.
- Noopur Raje; Shaji Kumar; Teru Hideshima; Aldo Roccaro; Kenji Ishitsuka; Hiroshi Yasui; Norihiko Shiraishi; Dharminder Chauhan; Nikhil C. Munshi; Simon R. Green; Kenneth C. Anderson (August 1, 2005). "Seliciclib (CYC202 or R-roscovitine), a small-molecule cyclin-dependent kinase inhibitor, mediates activity via down-regulation of Mcl-1 in multiple myeloma". Blood. 106 (3): 1042–1047. doi:10.1182/blood-2005-01-0320. PMC 1895150. PMID 15827128.
- Tomov, Nikola; Surchev, Lachezar; Wiedenmann, Clemens; Döbrössy, Máté; Nikkhah, Guido (August 2019). "Roscovitine, an experimental CDK5 inhibitor, causes delayed suppression of microglial, but not astroglial recruitment around intracerebral dopaminergic grafts". Experimental Neurology. 318: 135–144. doi:10.1016/j.expneurol.2019.04.013. PMID 31028828. S2CID 129946710.
- Menn, Bénédicte; Bach, Stéphane; Blevins, Teri L.; Campbell, Mark; Meijer, Laurent; Timsit, Serge (2010-08-12). Manzoni, Olivier Jacques (ed.). "Delayed Treatment with Systemic (S)-Roscovitine Provides Neuroprotection and Inhibits In Vivo CDK5 Activity Increase in Animal Stroke Models". PLOS ONE. 5 (8): e12117. Bibcode:2010PLoSO...512117M. doi:10.1371/journal.pone.0012117. ISSN 1932-6203. PMC 2920814. PMID 20711428.
- Rousselet, Estelle; Létondor, Anne; Menn, Bénédicte; Courbebaisse, Yann; Quillé, Marie-Lise; Timsit, Serge (June 2018). "Sustained (S)-roscovitine delivery promotes neuroprotection associated with functional recovery and decrease in brain edema in a randomized blind focal cerebral ischemia study". Journal of Cerebral Blood Flow & Metabolism. 38 (6): 1070–1084. doi:10.1177/0271678X17712163. ISSN 0271-678X. PMC 5998998. PMID 28569655.
- Pandey, Vimal; Ranjan, Nikhil; Narne, Parimala; Babu, Phanithi Prakash (2019-03-21). "Roscovitine effectively enhances antitumor activity of temozolomide in vitro and in vivo mediated by increased autophagy and Caspase-3 dependent apoptosis". Scientific Reports. 9 (1): 5012. Bibcode:2019NatSR...9.5012P. doi:10.1038/s41598-019-41380-1. ISSN 2045-2322. PMC 6428853. PMID 30899038.
- Sadaie MR, Mayner R, Doniger J (January 2004). "A novel approach to develop anti-HIV drugs: adapting non-nucleoside anticancer chemotherapeutics". Antiviral Research. 61 (1): 1–18. doi:10.1016/j.antiviral.2003.09.004. PMID 14670589.
- Pumfery A, de la Fuente C, Berro R, Nekhai S, Kashanchi F, Chao SH (2006). "Potential use of pharmacological cyclin-dependent kinase inhibitors as anti-HIV therapeutics". Curr Pharm Des. 12 (16): 1949–61. doi:10.2174/138161206777442083. PMID 16787240.
- Agbottah E, de La Fuente C, Nekhai S, Barnett A, Gianella-Borradori A, Pumfery A, Kashanchi F (28 January 2005). "Antiviral activity of CYC202 in HIV-1-infected cells". J. Biol. Chem. 280 (4): 3029–42. doi:10.1074/jbc.M406435200. PMID 15531588.
- Schang LM, Rosenberg A, Schaffer PA (2000). "Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins". J. Virol. 74 (5): 2107–20. doi:10.1128/JVI.74.5.2107-2120.2000. PMC 111691. PMID 10666240.
- Diwan P, Lacasse JJ, Schang LM (2004). "Roscovitine inhibits activation of promoters in herpes simplex virus type 1 genomes independently of promoter-specific factors". J. Virol. 78 (17): 9352–9365. doi:10.1128/JVI.78.17.9352-9365.2004. PMC 506918. PMID 15308730.
- Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, Gray M, Crescenzi E, Martin MC, Brady HJ, Savill JS, Dransfield I, Haslett C (2006). "Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis". Nature Medicine. 12 (9): 1056–1064. doi:10.1038/nm1468. PMID 16951685. S2CID 5875865.
- Doree M, Galas S (1994). "The cyclin-dependent protein kinases and the control of cell division". FASEB J. 8 (14): 1114–1121. doi:10.1096/fasebj.8.14.7958616. PMID 7958616. S2CID 3069118.