Umbralisib
Umbralisib, sold under the brand name Ukoniq, is a medication for the treatment of marginal zone lymphoma (MZL) and follicular lymphoma (FL).[2] It is taken by mouth.[2]
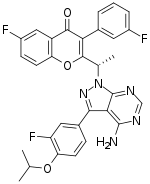 | |
| Clinical data | |
|---|---|
| Trade names | Ukoniq |
| Other names | RP5264; TGR-1202 |
| AHFS/Drugs.com | Ukoniq |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | CYP2C9, CYP3A4, and CYP1A2[1] |
| Elimination half-life | 91 h[1] |
| Excretion | Feces, urine[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C31H24F3N5O3 |
| Molar mass | 571.560 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Umbralisib is a kinase inhibitor including PI3K-delta and casein kinase CK1-epsilon.[2][4][5]
The most common side effects include increased creatinine, diarrhea-colitis, fatigue, nausea, neutropenia, transaminase elevation, musculoskeletal pain, anemia, thrombocytopenia, upper respiratory tract infection, vomiting, abdominal pain, decreased appetite, and rash.[2]
Umbralisib was granted accelerated approval for medical use in the United States in February 2021.[2][6][7] However, due to concerns for increased long term side effects leading to inferior overall survival which led to increased FDA scrutiny in the form of an ODAC review,[8] it has been withdrawn from the US market.[9]
Medical uses
On April 15, 2022, TG Therapeutics announced the voluntary withdrawal of Ukoniq (umbralisib) from sale for its approved use in the treatment of marginal zone lymphoma and follicular lymphoma. Furthermore, the company withdrew the pending Biologics License Application (BLA) and supplemental New Drug Application (sNDA) for the treatment of chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia (SLL) which utilized umbralisib in tandem with ublituximab, known as the "U2" regimen. The decision was based on the most recent overall survival (OS) data from the Phase 3 trial, Unity-CLL, that illustrated and increasing imbalance in OS.
Umbralisib was indicated for adults with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based regimen; and adults with relapsed or refractory follicular lymphoma (FL) who have received at least three prior lines of systemic therapy.[1][2][3][10]
Adverse effects
The prescribing information provides warnings and precautions for adverse reactions including infections, neutropenia, diarrhea and non-infectious colitis, hepatotoxicity, and severe cutaneous reactions.[2]
History
It has undergone clinical studies for chronic lymphocytic leukemia (CLL).[11][12] Three year data (including follicular lymphoma and DLBCL) was announced June 2016.[13] It is in combination trials for various leukemias and lymphomas, such as mantle cell lymphoma (MCL)[14][15] and other lymphomas.[16]
Umbralisib was granted breakthrough therapy designation by the U.S. Food and Drug Administration (FDA) for use in people with marginal zone lymphoma (MZL), a type of cancer with no specifically approved therapies.[17]
FDA approval was based on two single-arm cohorts of an open-label, multi-center, multi-cohort trial, UTX-TGR-205 (NCT02793583), in 69 participants with marginal zone lymphoma (MZL) who received at least one prior therapy, including an anti-CD20 containing regimen, and in 117 participants with follicular lymphoma (FL) after at least two prior systemic therapies.[2] The application for umbralisib was granted priority review for the marginal zone lymphoma (MZL) indication and orphan drug designation for the treatment of MZL and follicular lymphoma (FL).[2][18][19][20][21]
Society and culture
Legal status
In June 2022, due to safety concerns, the U.S. Food and Drug Administration (FDA) withdrew its approval for Ukoniq (umbralisib).[3]
Updated findings from the UNITY-CLL clinical trial show a possible increased risk of death in people receiving Ukoniq.[3] As a result, the FDA determined the risks of treatment with Ukoniq outweigh its benefits.[3] Based upon this determination, the drug's manufacturer, TG Therapeutics, announced it was voluntarily withdrawing Ukoniq from the market for the approved uses in MZL and FL.[3][22]
References
- "Ukoniq- umbralisib tablet, film coated". DailyMed. Retrieved 13 September 2021.
- "FDA grants accelerated approval to umbralisib for marginal zone lymphoma and follicular lymphoma". U.S. Food and Drug Administration (FDA). 5 February 2021. Retrieved 5 February 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA withdrawing cancer drug Ukoniq (umbralisib)". U.S. Food and Drug Administration (FDA). 1 June 2022. Retrieved 1 June 2022.
- Lunning M, Vose J, Nastoupil L, Fowler N, Burger JA, Wierda WG, et al. (November 2019). "Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia". Blood. 134 (21): 1811–20. doi:10.1182/blood.2019002118. PMC 7042665. PMID 31558467.
- Burris HA, Flinn IW, Patel MR, Fenske TS, Deng C, Brander DM, et al. (April 2018). "Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study". Lancet Oncology. 19 (4): 486–96. doi:10.1016/S1470-2045(18)30082-2. PMID 29475723.
- "TG Therapeutics Announces FDA Accelerated Approval of Ukoniq (umbralisib)" (Press release). TG Therapeutics. 5 February 2021. Retrieved 5 February 2021 – via GlobeNewswire.
- "Drug Approval Package: Ukoniq (umbralisib)". U.S. Food and Drug Administration (FDA). 5 March 2020. Retrieved 13 September 2021.
- https://www.fda.gov/media/157762/download
- "Federal Register :: Request Access".
- "TG Therapeutics Announces Voluntary Withdrawal of the BLA/sNDA for U2 to Treat Patients with CLL and SLL". TG Therapeutics. 15 April 2022.
- Inman S (19 March 2016). "Novel BTK, PI3K Inhibitors on Horizon for Relapsed CLL". OncLive. Archived from the original on 1 May 2016.
- "Therapy Focus –- TG Could Benefit From Zydelig Setback". Seeking Alpha. 29 March 2016.
- "TG Therapeutics, Inc. Announces First Patient Enrolled in the Registration-Directed UNITY-DLBCL Phase 2b Trial". TG Therapeutics Inc. June 2016.
- Clinical trial number NCT02268851 for "A Phase I/Ib Safety and Efficacy Study of the PI3K-delta Inhibitor TGR-1202 and Ibrutinib in Patients With CLL or MCL" at ClinicalTrials.gov
- "Follow-Up Data for Combination of TGR-1202 (umbralisib) plus Ibrutinib in Patients with Relapsed or Refractory CLL and MCL" (Press release). TG Therapeutics. 14 June 2017 – via Globenewswire.
- Clinical trial number NCT02793583 for "Study to Assess the Efficacy and Safety of Ublituximab + TGR-1202 With or Without Bendamustine and TGR-1202 Alone in Patients With Previously Treated Non-Hodgkin's Lymphoma (UNITY-NHL)" at ClinicalTrials.gov
- Columbus G (22 January 2019). "FDA Grants Umbralisib Breakthrough Designation for Marginal Zone Lymphoma". OncLive. Archived from the original on 23 January 2019.
- "Orphan Treatment of extranodal marginal zone lymphoma". U.S. Food and Drug Administration (FDA). 11 April 2019. Retrieved 5 February 2021.
- "Orphan Treatment of splenic marginal zone lymphoma". U.S. Food and Drug Administration (FDA). 11 April 2019. Retrieved 5 February 2021.
- "Orphan Treatment of Follicular Lymphoma". U.S. Food and Drug Administration (FDA). 11 April 2019. Retrieved 5 February 2021.
- "Orphan Treatment of nodal marginal zone lymphoma". U.S. Food and Drug Administration (FDA). 11 April 2019. Retrieved 5 February 2021.
- "TG Therapeutics Announces Voluntary Withdrawal of the BLA/sNDA for U2 to Treat Patients with CLL and SLL" (Press release). TG Therapeutics. 15 April 2022. Retrieved 1 June 2022.
External links
- "Umbralisib". Drug Information Portal. U.S. National Library of Medicine.
- "Umbralisib". NCI Drug Dictionary. National Cancer Institute.