Belzutifan
Belzutifan, sold under the brand name Welireg, is a medication used for the treatment of von Hippel–Lindau disease-associated renal cell carcinoma.[2][3][4][5][6][7] It is taken by mouth.[2]
 | |
| Clinical data | |
|---|---|
| Pronunciation | bell-ZOO-ti-fan |
| Trade names | Welireg |
| Other names | MK-6482, PT2977 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Antineoplastic |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
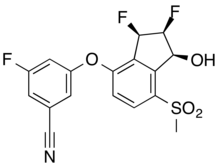
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C17H12F3NO4S |
| Molar mass | 383.34 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The most common side effects include decreased hemoglobin, anemia, fatigue, increased creatinine, headache, dizziness, increased glucose, and nausea.[3]
Belzutifan is an hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor.[2][3][8]
Belzutifan is the first drug to be awarded an "innovation passport" from the UK Medicines and Healthcare products Regulatory Agency (MHRA).[9][5] Belzutifan was approved for medical use in the United States in August 2021.[3][10] Belzutifan is the first hypoxia-inducible factor-2 alpha inhibitor therapy approved in the U.S.[10]
Medical uses
Belzutifan is indicated for treatment of adults with von Hippel-Lindau (VHL) disease who require therapy for associated renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, or pancreatic neuroendocrine tumors (pNET), not requiring immediate surgery.[3] Belzutifan was also found to be efficacious in an adolescent who had Pacak–Zhuang syndrome with polycythemia and paragangliomas.[11]
References
- https://pdf.hres.ca/dpd_pm/00066615.PDF
- "Welireg- belzutifan tablet, film coated". DailyMed. Retrieved 12 September 2021.
- "FDA approves belzutifan for cancers associated with von Hippel-Lindau". U.S. Food and Drug Administration (FDA). 13 August 2021. Retrieved 13 August 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Belzutifan". SPS - Specialist Pharmacy Service. 18 March 2021. Retrieved 25 April 2021.
- "MHRA awards first 'innovation passport' under new pathway". RAPS (Press release). Retrieved 25 April 2021.
- "Merck Receives Priority Review From FDA for New Drug Application for HIF-2α Inhibitor Belzutifan (MK-6482)" (Press release). Merck. 16 March 2016. Retrieved 25 April 2021 – via Business Wire.
- "FDA Grants Priority Review to Belzutifan for von Hippel-Lindau Disease–Associated RCC". Cancer Network. Retrieved 26 April 2021.
- Choueiri TK, Bauer TM, Papadopoulos KP, Plimack ER, Merchan JR, McDermott DF, et al. (April 2021). "Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis". Nat Med. 27 (5): 802–805. doi:10.1038/s41591-021-01324-7. PMC 9128828. PMID 33888901. S2CID 233371559.
- "First Innovation Passport awarded to help support development and access to cutting-edge medicines". Medicines and Healthcare products Regulatory Agency (MHRA) (Press release). 26 February 2021. Retrieved 14 August 2021.
- "FDA Approves Merck's Hypoxia-Inducible Factor-2 Alpha (HIF-2α) Inhibitor Welireg (belzutifan) for the Treatment of Patients With Certain Types of Von Hippel-Lindau (VHL) Disease-Associated Tumors" (Press release). Merck. 13 August 2021. Retrieved 13 August 2021 – via Business Wire.
- Kamihara, Junne; Hamilton, Kayla V.; Pollard, Jessica A.; Clinton, Catherine M.; Madden, Jill A.; Lin, Jasmine; Imamovic, Alma; Wall, Catherine B.; Wassner, Ari J.; Weil, Brent R.; Heeney, Matthew M. (25 November 2021). "Belzutifan, a Potent HIF2α Inhibitor, in the Pacak–Zhuang Syndrome". New England Journal of Medicine. 385 (22): 2059–2065. doi:10.1056/NEJMoa2110051. ISSN 0028-4793. PMID 34818480. S2CID 244651726.
External links
- "Belzutifan". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT04195750 for "A Study of Belzutifan (MK-6482) Versus Everolimus in Participants With Advanced Renal Cell Carcinoma (MK-6482-005)" at ClinicalTrials.gov
- Clinical trial number NCT03401788 for "A Phase 2 Study of Belzutifan (PT2977, MK-6482) for the Treatment of Von Hippel Lindau (VHL) Disease-Associated Renal Cell Carcinoma (RCC) (MK-6482-004)" at ClinicalTrials.gov