Alpelisib
Alpelisib, sold under the brand name Piqray among others, is a medication used to treat certain types of breast cancer.[7] It is used together with fulvestrant.[7] It is taken by mouth.[7] It is marketed by Novartis.[7]
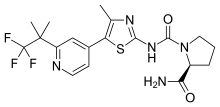 | |
| Clinical data | |
|---|---|
| Trade names | Piqray, Vijoice |
| Other names | BYL719 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619036 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.233.704 |
| Chemical and physical data | |
| Formula | C19H22F3N5O2S |
| Molar mass | 441.47 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Common side effects include high blood sugar, kidney problems, diarrhea, rash, low blood cells, liver problems, pancreatitis, vomiting, and hair loss.[7] It is an alpha-specific PI3K inhibitor.[7][8] It was approved for medical use in the United States in May 2019.[7][9]
Medical uses
Alpelisib is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.[10]
In the European Union, alpelisib is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)‑positive, human epidermal growth factor receptor 2 (HER2)‑negative, locally advanced or metastatic breast cancer with a PIK3CA mutation after disease progression following endocrine therapy as monotherapy.[6]
In April 2022, the indication for alpelisib was expanded in the US to include the treatment of severe manifestations of PIK3CA-related overgrowth spectrum (PROS) in those who require systemic therapy.[5][11][12]
History
In May 2019, alpelisib was approved in the United States for use in combination with the endocrine therapy fulvestrant, to treat postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer following progression on or after an endocrine-based regimen.[7][4][9]
The U.S. Food and Drug Administration (FDA) also approved the companion diagnostic test, therascreen PIK3CA RGQ PCR Kit, to detect the PIK3CA mutation in a tissue and/or a liquid biopsy.[7]
The efficacy of alpelisib was studied in the SOLAR-1 trial (NCT02437318), a randomized trial of 572 postmenopausal women and men with HR-positive, HER2-negative, advanced or metastatic breast cancer whose cancer had progressed while on or after receiving an aromatase inhibitor.[7][13]
The FDA granted the application for alpelisib priority review designation and granted approval of Piqray to Novartis.[7] The FDA granted approval of the therascreen PIK3CA RGQ PCR Kit to Qiagen Manchester, Ltd.[7]
On 28 May 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product alpelisib (Piqray), intended for the treatment of locally advanced or metastatic breast cancer with a PIK3CA mutation.[6] The applicant for this medicinal product is Novartis Europharm Limited.[6] Alpelisib was approved for medical use in the European Union in July 2020.[6]
Society and culture
References
- "Piqray Australian prescription medicine decision summary". Therapeutic Goods Administration (TGA). 27 March 2020. Archived from the original on 27 August 2021. Retrieved 16 August 2020.
- "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Archived from the original on 3 April 2022. Retrieved 13 May 2022.
- "Summary Basis of Decision (SBD) for Piqray". Health Canada. Archived from the original on 30 May 2022. Retrieved 29 May 2022.
- "Piqray- alpelisib tablet Piqray- alpelisib kit". DailyMed. 12 June 2020. Archived from the original on 28 October 2020. Retrieved 16 August 2020.
- "Vijoice- alpelisib tablet Vijoice- alpelisib kit". DailyMed. 7 April 2022. Archived from the original on 28 April 2022. Retrieved 27 April 2022.
- "Piqray EPAR". European Medicines Agency (EMA). 26 May 2020. Archived from the original on 14 August 2020. Retrieved 16 August 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "FDA approves first PI3K inhibitor for breast cancer". U.S. Food and Drug Administration (FDA) (Press release). 24 May 2019. Archived from the original on 25 November 2019. Retrieved 29 May 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. (May 2019). "PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer". The New England Journal of Medicine. 380 (20): 1929–1940. doi:10.1056/NEJMoa1813904. PMID 31091374.
- "Drug Approval Package: Piqray". U.S. Food and Drug Administration (FDA). 18 June 2019. Archived from the original on 25 November 2019. Retrieved 25 November 2019.
- "Piqray (alpelisib) tablets HR+/HER2- Advanced Breast Cancer Treatment". Novartis. Archived from the original on 24 August 2021. Retrieved 24 August 2021.
- "FDA approves alpelisib for PIK3CA-related overgrowth spectrum". U.S. Food and Drug Administration (FDA). 6 April 2022. Archived from the original on 7 April 2022. Retrieved 8 April 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves Novartis Vijoice (alpelisib) as first and only treatment for select patients with PIK3CA-Related Overgrowth Spectrum (PROS)". Novartis (Press release). 6 April 2022. Archived from the original on 6 April 2022. Retrieved 9 April 2022.
- "Drug Trials Snapshots: Piqray". U.S. Food and Drug Administration (FDA). 14 June 2019. Archived from the original on 25 November 2019. Retrieved 24 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "AusPAR: Alpelisib". Therapeutic Goods Administration (TGA). 3 September 2020. Archived from the original on 27 October 2020. Retrieved 23 September 2020.
External links
- "Alpelisib". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02437318 for "Study Assessing the Efficacy and Safety of Alpelisib Plus Fulvestrant in Men and Postmenopausal Women With Advanced Breast Cancer Which Progressed on or After Aromatase Inhibitor Treatment. (SOLAR-1)" at ClinicalTrials.gov