Rilpivirine
Rilpivirine, sold under the brand names Edurant and Rekambys, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS.[5][6] It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs such as efavirenz.[7][8]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Edurant, Rekambys |
| Other names | TMC278 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611037 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99.7% |
| Metabolism | CYP3A4 |
| Elimination half-life | tablets: 45 hours injection: 13–28 weeks |
| Excretion | 85% via faeces, 6% via urine |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.224.394 |
| Chemical and physical data | |
| Formula | C22H18N6 |
| Molar mass | 366.428 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Medical uses
In the US, rilpivirine is approved for treatment-naive patients with a viral load of 100,000 copies/mL or less at therapy initiation. It has to be combined with other drugs against HIV.[9]
In the European Union, rilpivirine is approved in combination with cabotegravir for maintenance treatment of adults who have undetectable HIV levels in the blood (viral load less than 50 copies/ml) with their current antiretroviral treatment, and when the virus has not developed resistance to certain class of anti-HIV medicines called non-nucleoside reverse transcriptase inhibitors (NNRTIs) and integrase strand transfer inhibitors (INIs).[4][10]
Available forms
The drug is available as tablets (brand name Edurant) and as a long-acting intramuscular injection to be given once every month or every two months (Rekambys). Before using the injection, the tablets are given for about four weeks to assess tolerability.[9][11]
Contraindications and interactions
The drug is contraindicated for use with drugs that induce the liver enzyme CYP3A4, such as carbamazepine, phenytoin, rifampicin, and St John's wort. Such drugs can accelerate the breaking down of rilpivirine, substantially decreasing its plasma concentrations and potentially resulting in loss of effectiveness and possible resistance.[9] Some of these drugs also induce the enzyme UGT1A1 and thus reduce blood plasma concentrations of cabotegravir, further compromising the effectiveness of this combination therapy.[12]
It is also contraindicated in combination with proton pump inhibitors because the increased gastric pH causes decreased rilpivirine absorption from the gut, with similar consequences as with CYP3A4 inducers.[9]
Adverse effects
The most common side effects of the injectable formulation are reactions at the injection site (in up to 84% of patients) such as pain and swelling, as well as headache (up to 12%) and fever or feeling hot (in 10%). Less common (under 10%) are depressive disorders, insomnia, and rashes.[9][11] The most common side effects of the tablets are also depressive disorders (4.1%), headache (3.5%), insomnia (3.5%) and rashes (2.3%).[13] All of these side effects occurred under combination therapies of rilpivirine with one or more other drugs against HIV.
QT prolongation of the heart rhythm has been observed at very high doses, but is not clinically relevant at standard doses of the drug.[13]
Pharmacology
Mechanism of action
Rilpivirine is a non-nucleoside reverse transcriptase inhibitor (NNRTI).[13]
Pharmacokinetics
When taken by mouth, rilpivirine reaches highest levels in the blood plasma after about four to five hours. Taking the drug without food lowers its plasma levels by 40% as compared to taking it with food, which is considered to be clinically relevant. Therefore, patients are advised to take the medication together with a meal.[13] After injection into the muscle, the substance reaches highest plasma levels after three to four days.[11]
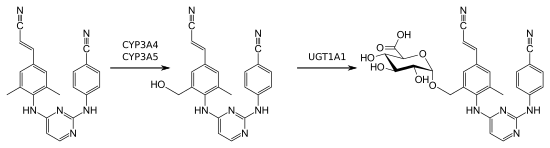
Independently of the mode of application, rilpivirine is almost completely bound to plasma proteins (99.7%), mostly to albumin. It is metabolised mainly by the liver enzyme CYP3A4. Metabolites include several oxidation products, glucuronides, and glucuronides of oxidized metabolites. The biological half-life is approximately 45 hours for the tablets and 13 to 28 weeks for the injection.[11][13]
Elimination has only been studied for oral administration: Most of the drug is excreted via the faeces (85%), partly in unchanged form (25%), partly in form of its metabolites (60%). A minor amount is excreted via the urine (6%), almost exclusively as metabolites.[11][13]
Fixed-dose combinations
A fixed-dose medication combining rilpivirine with emtricitabine and tenofovir disoproxil (TDF) was approved by the U.S. Food and Drug Administration (FDA) in August 2011 under the brand name Complera,[15] and was approved for use in the European Union with the brand name Eviplera in November 2011.[16] This combination has been shown to have higher rates of virologic failure than emtricitabine/tenofovir/efavirenz in people with baseline HIV viral loads greater than 100,000 copies/mm3.[17][18]
A fixed-dose medication combining rilpivirine with emtricitabine and tenofovir alafenamide (TAF) was approved for use in the US in March 2016 with the brand name Odefsey.[19]
Dolutegravir/rilpivirine, sold under the brand name Juluca, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It was approved for use in the United States in November 2017 and for use in the European Union in May 2018.
In January 2021, the U.S. Food and Drug Administration (FDA) approved cabotegravir/rilpivirine (brand name Cabenuva) for the treatment of HIV-1 infections in adults to replace a current antiretroviral regimen in those who are virologically suppressed on a stable antiretroviral regimen with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine.[20][21] This is the first FDA-approved injectable, complete regimen for HIV-infected adults that is administered once a month.[20][21] The label for rilpivirine tablets was revised to reflect the oral lead-in recommendations for use with cabotegravir.[21]
Chemistry
Like etravirine, a second-generation NNRTI approved in 2008, rilpivirine is a diarylpyrimidine (DAPY).[13]
The tablets contain rilpivirine hydrochloride,[13] while the injection contains free rilpivirine.[11]
History
Rilpivirine entered phase III clinical trials in April 2008,[22][23] and was approved for use in the United States in May 2011 under the brand name Edurant.[24][25]
On 15 October 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for rilpivirine under the trade name Rekambys, intended for the treatment of human immunodeficiency virus type-1 (HIV-1) infection in combination with cabotegravir injection.[26] It was approved for medical use in the European Union in December 2020.[4] The two medicines are the first antiretrovirals that come in a long-acting injectable formulation.[10]
References
- "Edurant 25 mg tablets - Summary of Product Characteristics (SmPC)". (emc). 21 January 2020. Retrieved 4 January 2021.
- "Edurant- rilpivirine hydrochloride tablet, film coated". DailyMed. Retrieved 4 January 2021.
- "Edurant EPAR". European Medicines Agency (EMA). Retrieved 4 January 2021.
- "Rekambys EPAR". European Medicines Agency (EMA). 13 October 2020. Retrieved 4 January 2021.
- "TMC278 — A new NNRTI". Tibotec. Archived from the original on 2008-12-20. Retrieved 2010-03-07.
- Stellbrink HJ (October 2007). "Antiviral drugs in the treatment of AIDS: what is in the pipeline ?". European Journal of Medical Research. 12 (9): 483–495. PMID 17933730.
- Goebel F, Yakovlev A, Pozniak AL, Vinogradova E, Boogaerts G, Hoetelmans R, et al. (August 2006). "Short-term antiviral activity of TMC278--a novel NNRTI--in treatment-naive HIV-1-infected subjects". AIDS. 20 (13): 1721–1726. doi:10.1097/01.aids.0000242818.65215.bd. PMID 16931936. S2CID 26078073.
- Pozniak A, Morales-Ramirez J, Mohap L, et al. "48-Week Primary Analysis of Trial TMC278-C204: TMC278 Demonstrates Potent and Sustained Efficacy in ART-naïve Patients. Oral abstract 144LB". 14th Conference on Retroviruses and Opportunistic Infections. Archived from the original on October 19, 2007.
- Rilpivirine Monograph. Accessed 2021-02-23.
- "First long-acting injectable antiretroviral therapy for HIV recommended approval". European Medicines Agency (EMA) (Press release). 16 October 2020. Retrieved 16 October 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Rekambys: EPAR – public assessment report" (PDF). European Medicines Agency. 2021-02-22.
- "Vocabria: EPAR – Product information" (PDF). European Medicines Agency. 2021-01-05.
- "Edurant: EPAR – public assessment report" (PDF). European Medicines Agency. 2021-01-04.
- Lade JM, Avery LB, Bumpus NN (October 2013). "Human biotransformation of the nonnucleoside reverse transcriptase inhibitor rilpivirine and a cross-species metabolism comparison". Antimicrobial Agents and Chemotherapy. 57 (10): 5067–5079. doi:10.1128/AAC.01401-13. PMC 3811466. PMID 23917319.
- "Approval of Complera: emtricitabine/rilpivirine/tenofovir DF fixed dose combination". U.S. Food and Drug Administration (FDA). August 10, 2011.
- "Eviplera". Aidsmap. Retrieved September 1, 2014.
- Haberfeld H, ed. (2021). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Eviplera Filmtabletten.
- Molina JM, Cahn P, Grinsztejn B, Lazzarin A, Mills A, Saag M, et al. (July 2011). "Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial". Lancet. 378 (9787): 238–246. doi:10.1016/S0140-6736(11)60936-7. PMID 21763936. S2CID 7313885.
- "Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide) Tablets". U.S. Food and Drug Administration (FDA). 29 November 2016. Retrieved 23 January 2021.
- "FDA Approves First Extended-Release, Injectable Drug Regimen for Adults Living with HIV". U.S. Food and Drug Administration (FDA) (Press release). 21 January 2021. Retrieved 21 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Cabenuva and Vocabria approved for HIV infection". U.S. Food and Drug Administration (FDA). 27 January 2021. Retrieved 27 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "A Clinical Trial in Treatment naïve HIV-1 Patients Comparing TMC278 to Efavirenz in Combination With Tenofovir + Emtricitabine". ClinicalTrials.gov. National Institutes of Health. October 25, 2012. Retrieved January 1, 2014.
- "A Clinical Trial in Treatment naïve HIV-Subjects Patients Comparing TMC278 to Efavirenz in Combination With 2 Nucleoside/Nucleotide Reverse Transcriptase Inhibitors". ClinicalTrials.gov. National Institutes of Health. May 14, 2012. Retrieved January 1, 2014.
- "Drug Approval Package: Edurant (rilpivirine) NDA #202022#". U.S. Food and Drug Administration (FDA). 20 August 2013. Retrieved 23 January 2021. Lay summary (PDF).
{{cite web}}: Cite uses deprecated parameter|lay-url=(help) - "FDA approves new HIV treatment" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on 2017-01-18. Retrieved 2011-05-20.
- "Rekambys: Pending EC decision". European Medicines Agency (EMA). 16 October 2020. Retrieved 16 October 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
- "Rilpivirine". Drug Information Portal. U.S. National Library of Medicine.
- "Rilpivirine hydrochloride". Drug Information Portal. U.S. National Library of Medicine.