Lenacapavir
Lenacapavir, sold under the brand name Sunlenca, is a medication used to treat HIV/AIDS.[1] It is taken by mouth or by subcutaneous injection.[1]
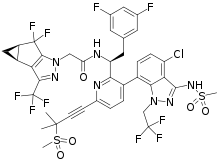 | |
| Clinical data | |
|---|---|
| Trade names | Sunlenca |
| Other names | GS-CA1, GS-6207 |
| Routes of administration | By mouth, subcutaneous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C39H32ClF10N7O5S2 |
| Molar mass | 968.28 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The most common side effects include reactions at the injection site and nausea.[1]
Lenacapavir was approved for medical use in the European Union in August 2022.[1]
History
Lenacapavir is being developed by Gilead Sciences.[2]
As of 2021, it is in phase II/III clinical trials.[3] It is being investigated as a treatment for HIV patients infected with multidrug-resistant virus and as a twice-yearly injectable for pre-exposure prophylaxis (PrEP).[3][4]
Society and culture
Legal status
On 23 June 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Sunlenca, intended for the treatment of adults with multidrug‑resistant human immunodeficiency virus type 1 (HIV‑1) infection.[5] The applicant for this medicinal product is Gilead Sciences Ireland UC.[5] Lenacapavir was approved for medical use in the European Union in August 2022.[1]
References
- "Sunlenca EPAR". European Medicines Agency (EMA). 22 June 2022. Archived from the original on 26 August 2022. Retrieved 25 August 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Link JO, Rhee MS, Tse WC, Zheng J, Somoza JR, Rowe W, et al. (August 2020). "Clinical targeting of HIV capsid protein with a long-acting small molecule". Nature. 584 (7822): 614–618. Bibcode:2020Natur.584..614L. doi:10.1038/s41586-020-2443-1. PMC 8188729. PMID 32612233. S2CID 220293679.
- Boerner H (11 March 2021). "Lenacapavir Effective in Multidrug Resistant HIV". Medscape. Archived from the original on 16 March 2021. Retrieved 15 March 2021.
- Highleyman L (15 March 2021). "Lenacapavir Shows Promise for Long-Acting HIV Treatment and Prevention". POZ. Archived from the original on 19 July 2021. Retrieved 15 March 2021.
- "Sunlenca: Pending EC decision". European Medicines Agency. 23 June 2022. Archived from the original on 26 June 2022. Retrieved 26 June 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
- "Lenacapavir". Drug Information Portal. U.S. National Library of Medicine.
- "Lenacapavir sodium". Drug Information Portal. U.S. National Library of Medicine.
- "Lenacapavir". Clinical Info. National Institutes of Health.