Head and neck cancer
Head and neck cancer develops from tissues in the lip and oral cavity (mouth), larynx (throat), salivary glands, nose, sinuses or the skin of the face.[5] The most common types of head and neck cancers occur in the lip, mouth, and larynx.[5] Symptoms predominantly include a sore that does not heal or a change in the voice.[1] Some may experience a sore throat that does not go away. In those with advanced disease, there may be unusual bleeding, facial pain, numbness or swelling, and visible lumps on the outside of the neck or oral cavity. Given the location of these cancers, trouble breathing may also be present.[6]
| Head and neck cancer | |
|---|---|
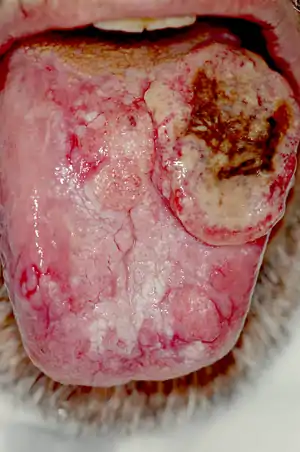 | |
| Extensive cancer of the tongue with surrounding lichen planus | |
| Specialty | Oncology, oral and maxillofacial surgery |
| Symptoms | Lump or sore that does not heal, sore throat that does not go away, trouble swallowing, change in the voice[1] |
| Risk factors | Alcohol, tobacco, betel quid, human papillomavirus, radiation exposure, certain workplace exposures, Epstein-Barr virus[1][2] |
| Diagnostic method | Tissue biopsy[1] |
| Prevention | Not using tobacco or alcohol[2] |
| Treatment | Surgery, radiation therapy, chemotherapy, targeted therapy[1] |
| Frequency | 5.5 million (affected during 2015)[3] |
| Deaths | 379,000 (2015)[4] |
The majority of head and neck cancer is caused by the use of alcohol or tobacco, including smokeless tobacco, with increasing cases linked to the human papillomavirus (HPV).[6][2] Other risk factors include Epstein-Barr virus, betel quid, radiation exposure, certain workplace exposures.[6] About 90% are pathologically classified as squamous cell cancers.[7][2] The diagnosis is confirmed by tissue biopsy.[6] The degree of surrounding tissue invasion and distant spread may be determined by medical imaging and blood tests.[6]
Not using tobacco or alcohol can reduce the risk for head and neck cancer.[2] The HPV vaccine may reduce the lifetime risk of oral cancer if taken prior to the onset of sexual activity, but confirmation will likely not be known until around 2060.[8] This is because oropharyngeal cancer typical presents in the 4th – 6th decade of life, and this is a relatively new vaccine. While screening in the general population does not appear to be useful, screening high risk groups by examination of the throat might be useful.[2] Head and neck cancer often is curable if it is diagnosed early; however, outcomes are typically poor if it is diagnosed late.[2] Treatment may include a combination of surgery, radiation therapy, chemotherapy, and targeted therapy.[6] Previous diagnosis and treatment of one head and neck cancer confers higher risk of developing a second head and neck cancer or recurrence.[6]
Globally, head and neck cancer accounts for 650,000 new cases of cancer and 330,000 deaths annually on average. In 2018, it was the seventh most common cancer worldwide with 890,000 new cases documented and 450,000 dying from the disease.[8] In the United States, head and neck cancer makes up 3% of all cancer cases (averaging 53,000 new diagnoses per year) and 1.5% of cancer deaths.[9] The 2017 worldwide figure cites head and neck cancers as representing 5.3% of all cancers (not including non-melanoma skin cancers).[10][5] Notably, head and neck cancer secondary to chronic alcohol or tobacco use has been steadily declining as less of the population chronically smokes tobacco.[8] However, HPV-associated oropharyngeal cancer is rising, particularly in younger people in westernized nations, which is thought to be reflective of changes in oral sexual practices, specifically with regard to the number of oral sexual partners.[5][8] This increase since the 1970s has mostly affected wealthier nations and male populations.[11][12][5] This is due to evidence suggesting that transmission rates of HPV from women to men are higher than men to women, as women often have a higher immune response to infection.[5][13]
The usual age at diagnosis is between 55 and 65 years old.[14] The average 5-year survival following diagnosis in the developed world is 42–64%.[14][15]
Signs and symptoms
Symptoms predominantly include a sore of the face or oral cavity that does not heal, trouble swallowing, or a change in the voice. In those with advanced disease, there may be unusual bleeding, facial pain, numbness or swelling, and visible lumps on the outside of the neck or oral cavity.[16] Head and neck cancer often begins with benign signs and symptoms of disease, like an enlarged lymph node on the outside of the neck, a hoarse-sounding voice or progressive worsening cough or sore throat. In the case of head and neck cancer, these symptoms will be notably persistent and become chronic. There may be a lump or a sore in the throat or neck that does not heal or go away. There may be difficult or painful swallowing. Speaking may become difficult. There may also be a persistent earache.[17]
Other symptoms can include: a lump in the lip, mouth or gums, ulcers or mouth sores that do not heal, bleeding from the mouth or numbness, bad breath, discolored patches that persist in the mouth, a sore tongue, and slurring of speech if the cancer is affecting the tongue. There may also be congested sinuses, weight loss, and some numbness or paralysis of facial muscles.
Mouth
_squamous_cell_carcinoma_histopathology.jpg.webp)
Squamous cell cancers are common in areas of the mouth, including the inner lip, tongue, floor of mouth, gums, and hard palate. Cancers of the mouth are strongly associated with tobacco use, especially use of chewing tobacco or dipping tobacco, as well as heavy alcohol use. Cancers of this region, particularly the tongue, are more frequently treated with surgery than are other head and neck cancers. Lip and oral cavity cancers are the most typically encountered type of head and neck cancers.[5]
Surgeries for oral cancers include:
- Maxillectomy (can be done with or without orbital exenteration)
- Mandibulectomy (removal of the lower jaw or part of it)
- Glossectomy (tongue removal, can be total, hemi or partial)
- Radical neck dissection
- Combinational e.g., glossectomy and laryngectomy done together.
The defect is typically covered/improved by using another part of the body and/or skin grafts and/or wearing a prosthesis.
Nose
Paranasal sinus and nasal cavity cancer affects the nasal cavity and the paranasal sinuses. Most of these cancers are squamous cell carcinomas.[18]
Nasopharynx
Nasopharyngeal cancer arises in the nasopharynx, the region in which the nasal cavities and the Eustachian tubes connect with the upper part of the throat. While some nasopharyngeal cancers are biologically similar to the common head and neck squamous cell carcinomas (HNSCCs), "poorly differentiated" nasopharyngeal carcinoma is lymphoepithelioma, which is distinct in its epidemiology, biology, clinical behavior, and treatment, and is treated as a separate disease by many experts.
Throat
Most oropharyngeal cancers are squamous cell carcinomas that begin in the oropharynx (throat), the middle part of the throat that includes the soft palate, the base of the tongue, and the tonsils.[1] Squamous cell cancers of the tonsils are more strongly associated with human papillomavirus infection than are cancers of other regions of the head and neck. HPV-positive oropharyngeal cancer generally has a better outcomes than HPV-negative disease with a 54% better survival,[19] but this advantage for HPV associated cancer applies only to oropharyngeal cancers.[20]
People with oropharyngeal carcinomas are at high risk of developing second primary head and neck cancer.[21]
Hypopharynx
The hypopharynx includes the pyriform sinuses, the posterior pharyngeal wall, and the postcricoid area. Tumors of the hypopharynx frequently have an advanced stage at diagnosis, and have the most adverse prognoses of pharyngeal tumors. They tend to metastasize early due to the extensive lymphatic network around the larynx.
Larynx
Laryngeal cancer begins in the larynx or "voice box", and is the second most common type of head and neck cancer encountered.[5] Cancer may occur on the vocal folds themselves ("glottic" cancer), or on tissues above and below the true cords ("supraglottic" and "subglottic" cancers respectively). Laryngeal cancer is strongly associated with tobacco smoking.
Surgery can include laser excision of small vocal cord lesions, partial laryngectomy (removal of part of the larynx) or total laryngectomy (removal of the whole larynx). If the whole larynx has been removed, the person is left with a permanent tracheostomy. Voice rehabilitation in such patients can be achieved through three important ways – esophageal speech, tracheoesophageal puncture, or electrolarynx. One would likely require the help of intensive teaching and speech therapy and/or an electronic device.
Trachea
Cancer of the trachea is a rare cancer usually classed as a lung cancer.[22]
Most tumors of the salivary glands differ from the common squamous cell carcinomas of the head and neck in cause, histopathology, clinical presentation, and therapy. Other uncommon tumors arising in the head and neck include teratomas, adenocarcinomas, adenoid cystic carcinomas, and mucoepidermoid carcinomas.[23] Rarer still are melanomas and lymphomas of the upper aerodigestive tract.
Causes
Alcohol and tobacco
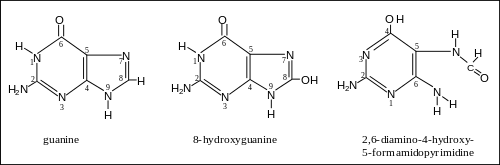
Around 75% of cases are caused by alcohol and tobacco use.[1]
Tobacco smoking is one of the main risk factors for head and neck cancer. A major carcinogenic compound in tobacco smoke is acrylonitrile.[24] Acrylonitrile appears to indirectly cause DNA damage by increasing oxidative stress, leading to increased levels of 8-oxo-2'-deoxyguanosine (8-oxo-dG) and formamidopyrimidine in DNA.[25] (see image). Both 8-oxo-dG and formamidopyrimidine are mutagenic.[26][27] DNA glycosylase NEIL1 prevents mutagenesis by 8-oxo-dG[28] and removes formamidopyrimidines from DNA.[29]
However, cigarette smokers have a lifetime increased risk for head and neck cancers that is 5- to 25-fold increased over the general population.[30] The ex-smoker's risk for developing a head and neck cancer begins to approach the risk in the general population 15 years after smoking cessation.[31] The high prevalence of tobacco and alcohol use worldwide and the high association of these cancers with these substances makes them ideal targets for enhanced cancer prevention.
Smokeless tobacco is a cause of oral cancer and oropharyngeal cancer.[32] Smokeless tobacco (including products where tobacco is chewed) is associated with a risk of developing head and neck cancers; this link has been established in the United States as well as in East Asian countries.[5][33][34]Cigar smoking is also an important risk factor for oral cancer.[35] It should also be noted that the use of electronic cigarettes may also lead to the development of head and neck cancers due to the substances like propylene glycol, glycerol, nitrosamines and metals contained there in; which can cause damage to airways.[36][5] This area of study requires more research to prove correlation and/or causation, however.[36][5]
Other environmental carcinogens suspected of being potential causes of head and neck cancer include occupational exposures such as nickel ore refining, exposure to textile fibers, and woodworking. Use of marijuana, especially when younger, has been linked to an increase in squamous-cell carcinoma cases in at least one study,[37] while other studies suggest use is not shown to be associated with oral squamous cell carcinoma, or associated with decreased squamous cell carcinoma.[38][39]
Diet
Excessive consumption of eggs, processed meats, and red meat were associated with increased rates of cancer of the head and neck in one study, while consumption of raw and cooked vegetables seemed to be protective.[40]
Vitamin E was not found to prevent the development of leukoplakia, the white plaques that are the precursor for carcinomas of the mucosal surfaces, in adult smokers.[41] Another study examined a combination of Vitamin E and beta carotene in smokers with early-stage cancer of the oropharynx, and found a worse prognosis in the vitamin users.[42]
Betel nut
Betel nut chewing is associated with an increased risk of squamous cell cancer of the head and neck.[1][43]
Human papillomavirus
Some head and neck cancers are caused by human papillomavirus (HPV).[1] In particular, HPV16 is a causal factor for some head and neck squamous-cell carcinomas (HNSCCs).[44][45] Approximately 15–25% of HNSCC contain genomic DNA from HPV,[46] and the association varies based on the site of the tumor,[47] especially HPV-positive oropharyngeal cancer, with highest distribution in the tonsils, where HPV DNA is found in (45 to 67%) of the cases,[48] less often in the hypopharynx (13–25%), and least often in the oral cavity (12–18%) and larynx (3–7%).[49][50]
Some experts estimate that while up to 50% of cancers of the tonsil may be infected with HPV, only 50% of these are likely to be caused by HPV (as opposed to the usual tobacco and alcohol causes). The role of HPV in the remaining 25–30% is not yet clear.[51] Oral sex is not risk free and results in a significant proportion of HPV-related head and neck cancer.[52]
Positive HPV16 status is associated with improved prognosis over HPV-negative OSCC.[53]
HPV can induce tumor by several mechanisms:[54]
- E6 and E7 oncogenic proteins.
- Disruption of tumor suppressor genes.
- High-level DNA amplifications, for example, oncogenes.
- Generating alternative nonfunctional transcripts.
- interchromosomal rearrangements.
- Distinct host genome methylation and expression patterns, produced even when virus isn't integrated into the host genome.
Induction of cancer can be associated for the expression of viral oncoproteins, the most important E6 and E7, or other mechanisms many of them run by the integration such as the generation of altered transcripts, disruption of tumor suppressors, high levels of DNA amplifications, interchromosomial rearrangements, or changes in DNA methylation patterns, the latter being able to find even when the virus is identified in episomes.[55][47] E6 sequesters p53 to promote p53 degradation while E7 inhibits pRb. p53 prevents cell growth when DNA is damaged by activating apoptosis and p21, a kinase that blocks the formation of cyclin D / Cdk4 avoiding pRb phosphorylation and thereby prevents release of E2F is a transcription factor required for activation of genes involved in cell proliferation. pRb remains bound to E2F while this action phosphorylated preventing activation of proliferation. Therefore, E6 and E7 act synergistically in triggering cell cycle progression and therefore uncontrolled proliferation by inactivating the p53 and Rb tumor suppressors.
Viral integration tends to occur in or near oncogenes or tumor suppressor genes and it is for this reason that the integration of the virus can greatly contribute to the development of tumor characteristics.[55]
Epstein–Barr virus
Epstein–Barr virus (EBV) infection is associated with nasopharyngeal cancer. Nasopharyngeal cancer occurs endemically in some countries of the Mediterranean and Asia, where EBV antibody titers can be measured to screen high-risk populations. Nasopharyngeal cancer has also been associated with consumption of salted fish, which may contain high levels of nitrites.[56]
Gastroesophageal reflux disease
The presence of acid reflux disease (gastroesophageal reflux disease [GERD]) or larynx reflux disease can also be a major factor. Stomach acids that flow up through the esophagus can damage its lining and raise susceptibility to throat cancer.
Hematopoietic stem cell transplantation
Patients after hematopoietic stem cell transplantation (HSCT) are at a higher risk for oral squamous cell carcinoma. Post-HSCT oral cancer may have more aggressive behavior with poorer prognosis, when compared to oral cancer in non-HSCT patients.[57] This effect is supposed to be owing to the continuous lifelong immune suppression and chronic oral graft-versus-host disease.[57]
Other possible causes
There are several risk factors for developing throat cancer. These include smoking or chewing tobacco or other things, such as gutkha, or paan, heavy alcohol consumption, poor diet resulting in vitamin deficiencies (worse if this is caused by heavy alcohol intake), weakened immune system, asbestos exposure, prolonged exposure to wood dust or paint fumes, exposure to petroleum industry chemicals, and being over the age of 55 years. Other risk factors include the appearance of white patches or spots in the mouth, known as leukoplakia,[23] which in about 1⁄3 of cases develops into cancer, and breathing or inhaling silica from cutting concrete, stone or cinder-blocks, especially in enclosed areas such as a warehouse, garage or basement.
Diagnosis

A person usually presents to the physician complaining of one or more of the above symptoms. The person will typically undergo a needle biopsy of this lesion, and a histopathologic information is available, a multidisciplinary discussion of the optimal treatment strategy will be undertaken between the radiation oncologist, surgical oncologist, and medical oncologist. Most (90%) cancers of the head and neck are squamous cell-derived termed as "head-and-neck squamous-cell carcinomas".[7]
Histopathology
Throat cancers are classified according to their histology or cell structure, and are commonly referred to by their location in the oral cavity and neck. This is because where the cancer appears in the throat affects the prognosis – some throat cancers are more aggressive than others depending upon their location. The stage at which the cancer is diagnosed is also a critical factor in the prognosis of throat cancer. Treatment guidelines recommend routine testing for the presence of HPV for all oropharyngeal squamous cell carcinoma tumours.[58]
Squamous-cell carcinoma
Squamous-cell carcinoma is a cancer of the squamous cell – a kind of epithelial cell found in both the skin and mucous membranes. It accounts for over 90% of all head and neck cancers,[59] including more than 90% of throat cancer.[23] Squamous cell carcinoma is most likely to appear in males over 40 years of age with a history of heavy alcohol use coupled with smoking.
The tumor marker Cyfra 21-1 may be useful in diagnosing squamous cell carcinoma of the head/neck (SCCHN).[60]
Adenocarcinoma
Adenocarcinoma is a cancer of epithelial tissue that has glandular characteristics. Several head and neck cancers are adenocarcinomas (either of intestinal or non-intestinal cell-type).[59]
Prevention
Avoidance of recognised risk factors (as described above) is the single most effective form of prevention. Regular dental examinations may identify pre-cancerous lesions in the oral cavity.[1]
When diagnosed early, oral, head and neck cancers can be treated more easily and the chances of survival increase tremendously.[1] As of 2017 it was not known if existing HPV vaccines can help prevent head and neck cancer.[1]
Management
Improvements in diagnosis and local management, as well as targeted therapy, have led to improvements in quality of life and survival for people with head and neck cancer.[61]
After a histologic diagnosis has been established and tumor extent determined, the selection of appropriate treatment for a specific cancer depends on a complex array of variables, including tumor site, relative morbidity of various treatment options, concomitant health problems, social and logistic factors, previous primary tumors, and the person's preference. Treatment planning generally requires a multidisciplinary approach involving specialist surgeons and medical and radiation oncologists.
Surgical resection and radiation therapy are the mainstays of treatment for most head and neck cancers and remain the standard of care in most cases. For small primary cancers without regional metastases (stage I or II), wide surgical excision alone or curative radiation therapy alone is used. More extensive primary tumors, or those with regional metastases (stage III or IV), planned combinations of pre- or postoperative radiation and complete surgical excision are generally used. More recently, as historical survival and control rates are recognized as less than satisfactory, there has been an emphasis on the use of various induction or concomitant chemotherapy regimens.
Surgery
Surgery as a treatment is frequently used in most types of head and neck cancer. Usually the goal is to remove the cancerous cells entirely. This can be particularly tricky if the cancer is near the larynx and can result in the person being unable to speak. Surgery is also commonly used to resect (remove) some or all of the cervical lymph nodes to prevent further spread of the disease.
CO2 laser surgery is also another form of treatment. Transoral laser microsurgery allows surgeons to remove tumors from the voice box with no external incisions. It also allows access to tumors that are not reachable with robotic surgery. During the surgery, surgeon and pathologist work together to assess the adequacy of excision ("margin status"), minimizing the amount of normal tissue removed or damaged.[62] This technique helps give the person as much speech and swallowing function as possible after surgery.[63]
Radiation therapy

Radiation therapy is the most common form of treatment. There are different forms of radiation therapy, including 3D conformal radiation therapy, intensity-modulated radiation therapy, particle beam therapy and brachytherapy, which are commonly used in the treatments of cancers of the head and neck. Most people with head and neck cancer who are treated in the United States and Europe are treated with intensity-modulated radiation therapy using high energy photons. At higher doses, head and neck radiation is associated with thyroid dysfunction and pituitary axis dysfunction.[64] Radiation therapy of head and neck cancers can also cause acute skin reactions of varying levels of severity, which can be treated and managed with topically applied creams or specialist films.[65]
Chemotherapy
Chemotherapy in throat cancer is not generally used to cure the cancer as such. Instead, it is used to provide an inhospitable environment for metastases so that they will not establish in other parts of the body. Typical chemotherapy agents are a combination of paclitaxel and carboplatin. Cetuximab is also used in the treatment of throat cancer.
Docetaxel-based chemotherapy has shown a very good response in locally advanced head and neck cancer. Docetaxel is the only taxane approved by US FDA for head and neck cancer, in combination with cisplatin and fluorouracil for the induction treatment of inoperable, locally advanced squamous cell carcinoma of the head and neck.[66]
While not specifically a chemotherapy, amifostine is often administered intravenously by a chemotherapy clinic prior to IMRT radiotherapy sessions. Amifostine protects the gums and salivary glands from the effects of radiation.
There is no evidence that erythropoietin should be routinely given with radiotherapy.[67]
Photodynamic therapy
Photodynamic therapy may have promise in treating mucosal dysplasia and small head and neck tumors.[23] Amphinex is giving good results in early clinical trials for treatment of advanced head and neck cancer.[68]
Targeted therapy
Targeted therapy, according to the National Cancer Institute, is "a type of treatment that uses drugs or other substances, such as monoclonal antibodies, to identify and attack specific cancer cells without harming normal cells." Some targeted therapy used in squamous cell cancers of the head and neck include cetuximab, bevacizumab and erlotinib.
The best quality data are available for cetuximab since the 2006 publication of a randomized clinical trial comparing radiation treatment plus cetuximab versus radiation treatment alone.[69] This study found that concurrent cetuximab and radiotherapy improves survival and locoregional disease control compared to radiotherapy alone, without a substantial increase in side effects, as would be expected with the concurrent chemoradiotherapy, which is the current gold standard treatment for advanced head and neck cancer. Whilst this study is of pivotal significance, interpretation is difficult since cetuximab-radiotherapy was not directly compared to chemoradiotherapy. The results of ongoing studies to clarify the role of cetuximab in this disease are awaited with interest.
Another study evaluated the impact of adding cetuximab to conventional chemotherapy (cisplatin) versus cisplatin alone. This study found no improvement in survival or disease-free survival with the addition of cetuximab to the conventional chemotherapy.[70]
However, another study which completed in March 2007 found that there was an improvement in survival.
A 2010 review concluded that the combination of cetuximab and platin/5-fluorouracil should be considered the current standard first-line regimen.[71]
Gendicine is a gene therapy that employs an adenovirus to deliver the tumor suppressor gene p53 to cells. It was approved in China in 2003 for the treatment of head and neck squamous cell carcinoma.[72]
The mutational profile of HPV+ and HPV- head and neck cancer has been reported, further demonstrating that they are fundamentally distinct diseases. [73]
Immunotherapy
Immunotherapy is a type of treatment that activates the immune system to fight cancer. One type of immunotherapy, immune checkpoint blockade, binds to and blocks inhibitory signals on immune cells to release their anti-cancer activities.
In 2016, the FDA granted accelerated approval to pembrolizumab for the treatment of people with recurrent or metastatic HNSCC with disease progression on or after platinum-containing chemotherapy.[74] Later that year, the FDA approved nivolumab for the treatment of recurrent or metastatic HNSCC with disease progression on or after platinum-based chemotherapy.[75] In 2019, the FDA approved pembrolizumab for the first-line treatment of metastatic or unresectable recurrent HNSCC.[76]
Treatment side effects
Depending on the treatment used, people with head and neck cancer may experience the following symptoms and treatment side effects:[23][65]
- Eating problems
- Pain associated with lesions
- Mucositis
- Nephrotoxicity and ototoxicity
- Xerostomia
- Gastroesophageal reflux
- Radiation-induced osteonecrosis of the jaw
- Radiation-induced acute skin reactions
Psychosocial
Programs to support the emotional and social well-being of people who have been diagnosed with head and neck cancer may be offered.[77] There is no clear evidence on the effectiveness of these interventions or any particular type of psychosocial program or length of time that is the most helpful for those with head and neck cancer.[77]
Prognosis
Although early-stage head and neck cancers (especially laryngeal and oral cavity) have high cure rates, up to 50% of people with head and neck cancer present with advanced disease.[78] Cure rates decrease in locally advanced cases, whose probability of cure is inversely related to tumor size and even more so to the extent of regional node involvement. HPV-associated oropharyngeal cancer has been shown to respond better to chemoradiation and, subsequently, a better prognosis, compared to non-associated HPV head and neck cancer.[8]
Consensus panels in America (AJCC) and Europe (UICC) have established staging systems for head and neck squamous-cell cancers. These staging systems attempt to standardize clinical trial criteria for research studies, and attempt to define prognostic categories of disease. Squamous cell cancers of the head and neck are staged according to the TNM classification system, where T is the size and configuration of the tumor, N is the presence or absence of lymph node metastases, and M is the presence or absence of distant metastases. The T, N, and M characteristics are combined to produce a "stage" of the cancer, from I to IVB.[79]
Problem of second primaries
Survival advantages provided by new treatment modalities have been undermined by the significant percentage of people cured of head and neck squamous cell carcinoma (HNSCC) who subsequently develop second primary tumors. The incidence of second primary tumors ranges in studies from 9%[80] to 23%[81] at 20 years. Second primary tumors are the major threat to long-term survival after successful therapy of early-stage HNSCC.[82] Their high incidence results from the same carcinogenic exposure responsible for the initial primary process, called field cancerization.
Digestive system
Many people with head and neck cancer are also not able to eat sufficiently. A tumor may impair a person's ability to swallow and eat, and throat cancer may affect the digestive system. The difficulty in swallowing can lead to a person to choke on their food in the early stages of digestion and interfere with the food's smooth travels down into the esophagus and beyond.
The treatments for throat cancer can also be harmful to the digestive system as well as other body systems. Radiation therapy can lead to nausea and vomiting, which can deprive a body of vital fluids (although these may be obtained through intravenous fluids if necessary). Frequent vomiting can lead to an electrolyte imbalance which has serious consequences for the proper functioning of the heart. Frequent vomiting can also upset the balance of stomach acids which has a negative impact on the digestive system, especially the lining of the stomach and esophagus.
Enteral feeding, a method that adds nutrients directly into a person's stomach using a nasogastric feeding tube or a gastrostomy tube, may be necessary for some people.[83] Further research is required to determine the most effective method of enteral feeding to ensure that people undergoing radiotherapy or chemoradiation treatment are able to stay nourished during their treatment.[83]
Respiratory system
In the cases of some throat cancers, the air passages in the mouth and behind the nose may become blocked from lumps or the swelling from the open sores. If the throat cancer is near the bottom of the throat, it has a high likelihood of spreading to the lungs and interfering with the person's ability to breathe; this is even more likely if the person is a smoker, because they are highly susceptible to lung cancer.
Mental health
Cancer in the head or neck may impact a person's mental well-being and can sometimes lead to social isolation.[77] This largely results from decreased ability or inability to eat, speak or effectively communicate. Physical appearance is often altered by both the cancer itself or as a consequence of treatment side effects. Psychological distress may occur and feelings such as uncertainty and fear may arise.[77] Some people may also have a changed physical appearance, differences in swallowing or breathing, and residual pain to manage.[77]
Patient Experience
Increased Caregiver Distress Unique to care for Head and Neck Cancer Patients
Multiple studies have shown caregivers for head and neck cancer patients show higher rates of distress and poorer mental health compared to both the general population as well as those caring for non-head-and-neck cancer patients.[84] The high symptom burden patients' experience necessitate complex caregiver roles, often requiring hospital staff training, which caregivers can find distressing when asked to do so for the first time. It is becoming increasingly apparent caregivers (most often spouses, children or close family members) might not be adequately informed about, nor prepared or trained for the tasks and roles he/she will encounter during the treatment and recovery phase for this unique patient population, which span both technical and emotional support.[85] Of note, caregivers of patient's who report lower quality of life, demonstrate increased burden and fatigue that extents beyond the treatment phase.
Examples of technically difficult caregiver duties include: tube feeding, oral suctioning, wound maintenance, medication delivery safe for tube feeding, and trouble shooting at home medical equipment. If the cancer affect the mouth or larynx, caregivers must also find a way to effectively communicate amongst themselves and with their healthcare team. This is in addition to providing emotional support for the person undergoing cancer therapy.[85]
Others
Like any cancer, metastasization affects many areas of the body, as the cancer spreads from cell to cell and organ to organ. For example, if it spreads to the bone marrow, it will prevent the body from producing enough red blood cells and affects the proper functioning of the white blood cells and the body's immune system; spreading to the circulatory system will prevent oxygen from being transported to all the cells of the body; and throat cancer can throw the nervous system into chaos, making it unable to properly regulate and control the body.
Epidemiology

The number of new cases of head and neck cancers in the United States was 40,490 in 2006, accounting for about 3% of adult malignancies. A total of 11,170 people died of their disease in 2006.[87] The worldwide incidence exceeds half a million cases annually. In North America and Europe, the tumors usually arise from the oral cavity, oropharynx, or larynx, whereas nasopharyngeal cancer is more common in the Mediterranean countries and in the Far East. In Southeast China and Taiwan, head and neck cancer, specifically nasopharyngeal cancer, is the most-common cause of death in young men.[88]
- In 2008, there were 22,900 cases of oral cavity cancer, 12,250 cases of laryngeal cancer, and 12,410 cases of pharyngeal cancer in the United States.[23]
- In 2002, 7,400 Americans were projected to die of these cancers.[89]
- More than 70% of throat cancers are at an advanced stage when discovered.[90]
- Men are 89% more likely than women to be diagnosed with, and are almost twice as likely to die of these cancers.[89]
- African Americans are disproportionately affected by head and neck cancer, with younger ages of incidence, increased mortality, and more advanced disease at presentation.[78] Laryngeal cancer incidence is higher in African Americans relative to white, Asian, and Hispanic populations. There is a lower survival rate for similar tumor states in African Americans with head and neck cancer.[23]
- Smoking and tobacco use are directly related to oropharyngeal (throat) cancer deaths.[91]
- The risk of developing head and neck cancers increases with age, especially after 50 years. Most people who do so are between 50 and 70 years old.[23]
Research
Immunotherapy with immune checkpoint inhibitors is being investigated in head and neck cancers.[92]
References
- "Oropharyngeal Cancer Treatment (Adult) (PDQ®)–Patient Version". National Cancer Institute. 22 November 2019. Retrieved 28 November 2019.
- World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.8. ISBN 978-9283204299.
- Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality and Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- Aupérin A (May 2020). "Epidemiology of head and neck cancers: an update". Current Opinion in Oncology. 32 (3): 178–186. doi:10.1097/CCO.0000000000000629. PMID 32209823. S2CID 214644380.
- "Head and Neck Cancers". NCI. 29 March 2017. Retrieved 7 February 2021.
- Vigneswaran N, Williams MD (May 2014). "Epidemiologic trends in head and neck cancer and aids in diagnosis". Oral and Maxillofacial Surgery Clinics of North America. 26 (2): 123–141. doi:10.1016/j.coms.2014.01.001. PMC 4040236. PMID 24794262.
- Chow LQ (January 2020). "Head and Neck Cancer". The New England Journal of Medicine. 382 (1): 60–72. doi:10.1056/nejmra1715715. PMID 31893516. S2CID 209482428.
- Siegel RL, Miller KD, Jemal A (January 2020). "Cancer statistics, 2020". CA: A Cancer Journal for Clinicians. 70 (1): 7–30. doi:10.3322/caac.21590. PMID 31912902.
- Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. (Global Burden of Disease Cancer Collaboration) (December 2019). "Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study". JAMA Oncology. 5 (12): 1749–1768. doi:10.1001/jamaoncol.2019.2996. PMC 6777271. PMID 31560378.
- Gillison ML, Castellsagué X, Chaturvedi A, Goodman MT, Snijders P, Tommasino M, et al. (February 2014). "Eurogin Roadmap: comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix". International Journal of Cancer. 134 (3): 497–507. doi:10.1002/ijc.28201. PMID 23568556. S2CID 37877664.
- Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C (October 2015). "Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma". Journal of Clinical Oncology. 33 (29): 3235–3242. doi:10.1200/JCO.2015.61.6995. PMC 4979086. PMID 26351338.
- Giuliano AR, Nyitray AG, Kreimer AR, Pierce Campbell CM, Goodman MT, Sudenga SL, et al. (June 2015). "EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection". International Journal of Cancer. 136 (12): 2752–2760. doi:10.1002/ijc.29082. PMC 4297584. PMID 25043222.
- "SEER Stat Fact Sheets: Oral Cavity and Pharynx Cancer". SEER. April 2016. Archived from the original on 15 November 2016. Retrieved 29 September 2016.
- Beyzadeoglu M, Ozyigit G, Selek U (2014). Radiation Therapy for Head and Neck Cancers: A Case-Based Review. Springer. p. 18. ISBN 9783319104133. Archived from the original on 2017-09-10.
- McIlwain WR, Sood AJ, Nguyen SA, Day TA (May 2014). "Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer". JAMA Otolaryngology–Head & Neck Surgery. 140 (5): 441–447. doi:10.1001/jamaoto.2014.141. PMID 24652023.
- Ensley JF (2003). Head and neck cancer : emerging perspectives. Amsterdam: Academic Press. ISBN 978-0-08-053384-1. OCLC 180905431.
- "Paranasal Sinus and Nasal Cavity Cancer Treatment (Adult) (PDQ®)–Patient Version". National Cancer Institute. 8 November 2019. Retrieved 4 December 2019.
- O'Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA (December 2012). "Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis". Oral Oncology. 48 (12): 1191–1201. doi:10.1016/j.oraloncology.2012.06.019. PMID 22841677. Archived (PDF) from the original on 2017-09-10.
- Ragin CC, Taioli E (October 2007). "Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis". International Journal of Cancer. 121 (8): 1813–1820. doi:10.1002/ijc.22851. PMID 17546592.
- Krishnatreya M, Rahman T, Kataki AC, Das A, Das AK, Lahkar K (2013). "Synchronous primary cancers of the head and neck region and upper aero digestive tract: defining high-risk patients". Indian Journal of Cancer. 50 (4): 322–326. doi:10.4103/0019-509x.123610. PMID 24369209.
- "Throat cancer | Head and neck cancers | Cancer Research UK". www.cancerresearchuk.org. Retrieved 28 November 2019.
- Ridge JA, Glisson BS, Lango MN, Feigenberg S, Horwitz EM (2008). "Head and neck tumors." (PDF). In Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (eds.). Cancer management: a multidisciplinary approach (11th ed.). pp. 39–86.
- Cunningham FH, Fiebelkorn S, Johnson M, Meredith C (November 2011). "A novel application of the Margin of Exposure approach: segregation of tobacco smoke toxicants". Food and Chemical Toxicology. 49 (11): 2921–2933. doi:10.1016/j.fct.2011.07.019. PMID 21802474.
- Pu X, Kamendulis LM, Klaunig JE (September 2009). "Acrylonitrile-induced oxidative stress and oxidative DNA damage in male Sprague-Dawley rats". Toxicological Sciences. 111 (1): 64–71. doi:10.1093/toxsci/kfp133. PMC 2726299. PMID 19546159.
- Kalam MA, Haraguchi K, Chandani S, Loechler EL, Moriya M, Greenberg MM, Basu AK (2006). "Genetic effects of oxidative DNA damages: comparative mutagenesis of the imidazole ring-opened formamidopyrimidines (Fapy lesions) and 8-oxo-purines in simian kidney cells". Nucleic Acids Research. 34 (8): 2305–2315. doi:10.1093/nar/gkl099. PMC 1458282. PMID 16679449.
- Jena NR, Mishra PC (October 2013). "Is FapyG mutagenic?: Evidence from the DFT study". ChemPhysChem. 14 (14): 3263–3270. doi:10.1002/cphc.201300535. PMID 23934915.
- Suzuki T, Harashima H, Kamiya H (May 2010). "Effects of base excision repair proteins on mutagenesis by 8-oxo-7,8-dihydroguanine (8-hydroxyguanine) paired with cytosine and adenine". DNA Repair. 9 (5): 542–550. doi:10.1016/j.dnarep.2010.02.004. hdl:2115/43021. PMID 20197241. S2CID 207147128.
- Nemec AA, Wallace SS, Sweasy JB (October 2010). "Variant base excision repair proteins: contributors to genomic instability". Seminars in Cancer Biology. 20 (5): 320–328. doi:10.1016/j.semcancer.2010.10.010. PMC 3254599. PMID 20955798.
- Andre K, Schraub S, Mercier M, Bontemps P (September 1995). "Role of alcohol and tobacco in the aetiology of head and neck cancer: a case-control study in the Doubs region of France". European Journal of Cancer, Part B. 31B (5): 301–309. doi:10.1016/0964-1955(95)00041-0. PMID 8704646.
- La Vecchia C, Franceschi S, Bosetti C, Levi F, Talamini R, Negri E (April 1999). "Time since stopping smoking and the risk of oral and pharyngeal cancers". Journal of the National Cancer Institute. 91 (8): 726–728. doi:10.1093/jnci/91.8.726a. hdl:2434/520105. PMID 10218516.
- Winn D (1992). "Smokeless tobacco and aerodigestive tract cancers: recent research directions". The Biology and Prevention of Aerodigestive Tract Cancers. Adv Exp Med Biol. Advances in Experimental Medicine and Biology. Vol. 320. pp. 39–46. doi:10.1007/978-1-4615-3468-6_6. ISBN 978-0-306-44244-5. PMID 1442283.
- Wyss AB, Hashibe M, Lee YA, Chuang SC, Muscat J, Chen C, et al. (November 2016). "Smokeless Tobacco Use and the Risk of Head and Neck Cancer: Pooled Analysis of US Studies in the INHANCE Consortium". American Journal of Epidemiology. 184 (10): 703–716. doi:10.1093/aje/kww075. PMC 5141945. PMID 27744388.
- Lee YA, Li S, Chen Y, Li Q, Chen CJ, Hsu WL, et al. (January 2019). "Tobacco smoking, alcohol drinking, betel quid chewing, and the risk of head and neck cancer in an East Asian population". Head & Neck. 41 (1): 92–102. doi:10.1002/hed.25383. PMID 30552826. S2CID 54632009.
- Iribarren C, Tekawa IS, Sidney S, Friedman GD (June 1999). "Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men". The New England Journal of Medicine. 340 (23): 1773–1780. CiteSeerX 10.1.1.460.1056. doi:10.1056/NEJM199906103402301. PMID 10362820.
- Ralho A, Coelho A, Ribeiro M, Paula A, Amaro I, Sousa J, et al. (December 2019). "Effects of Electronic Cigarettes on Oral Cavity: A Systematic Review". The Journal of Evidence-Based Dental Practice. 19 (4): 101318. doi:10.1016/j.jebdp.2019.04.002. PMID 31843181. S2CID 145920823.
- Zhang ZF, Morgenstern H, Spitz MR, Tashkin DP, Yu GP, Marshall JR, et al. (December 1999). "Marijuana use and increased risk of squamous cell carcinoma of the head and neck". Cancer Epidemiology, Biomarkers & Prevention. 8 (12): 1071–1078. PMID 10613339.
- Rosenblatt KA, Daling JR, Chen C, Sherman KJ, Schwartz SM (June 2004). "Marijuana use and risk of oral squamous cell carcinoma". Cancer Research. 64 (11): 4049–4054. doi:10.1158/0008-5472.CAN-03-3425. PMID 15173020.
- Liang C, McClean MD, Marsit C, Christensen B, Peters E, Nelson HH, Kelsey KT (August 2009). "A population-based case-control study of marijuana use and head and neck squamous cell carcinoma". Cancer Prevention Research. 2 (8): 759–768. doi:10.1158/1940-6207.CAPR-09-0048. PMC 2812803. PMID 19638490.
- Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S, Monnier P (August 1998). "Food groups and risk of oral and pharyngeal cancer". International Journal of Cancer. 77 (5): 705–709. doi:10.1002/(SICI)1097-0215(19980831)77:5<705::AID-IJC8>3.0.CO;2-Z. PMID 9688303.
- Liede K, Hietanen J, Saxen L, Haukka J, Timonen T, Häyrinen-Immonen R, Heinonen OP (June 1998). "Long-term supplementation with alpha-tocopherol and beta-carotene and prevalence of oral mucosal lesions in smokers". Oral Diseases. 4 (2): 78–83. doi:10.1111/j.1601-0825.1998.tb00261.x. PMID 9680894.
- Bairati I, Meyer F, Gélinas M, Fortin A, Nabid A, Brochet F, et al. (April 2005). "A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients". Journal of the National Cancer Institute. 97 (7): 481–488. doi:10.1093/jnci/dji095. PMID 15812073.
- Jeng JH, Chang MC, Hahn LJ (September 2001). "Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives". Oral Oncology. 37 (6): 477–492. doi:10.1016/S1368-8375(01)00003-3. PMID 11435174.
- "Biomarkers for Cancers of the Head and Neck". Retrieved 2014-08-07.
- D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. (May 2007). "Case-control study of human papillomavirus and oropharyngeal cancer". The New England Journal of Medicine. 356 (19): 1944–1956. doi:10.1056/NEJMoa065497. PMID 17494927. S2CID 18819678.
- Kreimer AR, Clifford GM, Boyle P, Franceschi S (February 2005). "Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review". Cancer Epidemiology, Biomarkers & Prevention. 14 (2): 467–475. doi:10.1158/1055-9965.EPI-04-0551. PMID 15734974.
- Joseph AW, D'Souza G (August 2012). "Epidemiology of human papillomavirus-related head and neck cancer". Otolaryngologic Clinics of North America. 45 (4): 739–764. doi:10.1016/j.otc.2012.04.003. PMID 22793850.
- Perez-Ordoñez B, Beauchemin M, Jordan RC (May 2006). "Molecular biology of squamous cell carcinoma of the head and neck". Journal of Clinical Pathology. 59 (5): 445–453. doi:10.1136/jcp.2003.007641. PMC 1860277. PMID 16644882.
- Paz IB, Cook N, Odom-Maryon T, Xie Y, Wilczynski SP (February 1997). "Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer's tonsillar ring". Cancer. 79 (3): 595–604. doi:10.1002/(SICI)1097-0142(19970201)79:3<595::AID-CNCR24>3.0.CO;2-Y. PMID 9028373.
- Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, Thomas SJ (August 2006). "Human papillomavirus and head and neck cancer: a systematic review and meta-analysis" (PDF). Clinical Otolaryngology. 31 (4): 259–266. doi:10.1111/j.1749-4486.2006.01246.x. PMID 16911640. S2CID 2502403. Archived (PDF) from the original on 2017-08-11.
- Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. (February 2006). "Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis". Journal of Clinical Oncology. 24 (5): 736–747. doi:10.1200/JCO.2004.00.3335. PMID 16401683. Archived from the original on 2009-01-20.
- Haddad RI (2007). "Human Papillomavirus Infection and Oropharyngeal Cancer" (PDF). Archived from the original (PDF) on 2012-05-13. Retrieved 2012-09-26.
- Tahtali A, Hey C, Geissler C, Filman N, Diensthuber M, Leinung M, et al. (August 2013). "HPV status and overall survival of patients with oropharyngeal squamous cell carcinoma--a retrospective study of a German head and neck cancer center". Anticancer Research. 33 (8): 3481–3485. PMID 23898123. Archived from the original on 2016-01-07.
- Schmitz M, Driesch C, Beer-Grondke K, Jansen L, Runnebaum IB, Dürst M (September 2012). "Loss of gene function as a consequence of human papillomavirus DNA integration". International Journal of Cancer. 131 (5): E593–E602. doi:10.1002/ijc.27433. PMID 22262398. S2CID 21515048.
- Parfenov M, Pedamallu CS, Gehlenborg N, Freeman SS, Danilova L, Bristow CA, et al. (October 2014). "Characterization of HPV and host genome interactions in primary head and neck cancers". Proceedings of the National Academy of Sciences of the United States of America. 111 (43): 15544–15549. Bibcode:2014PNAS..11115544P. doi:10.1073/pnas.1416074111. PMC 4217452. PMID 25313082.
- "Risks and causes | Nasopharyngeal cancer | Cancer Research UK". www.cancerresearchuk.org. Retrieved 4 December 2019.
- Elad S, Zadik Y, Zeevi I, Miyazaki A, de Figueiredo MA, Or R (December 2010). "Oral cancer in patients after hematopoietic stem-cell transplantation: long-term follow-up suggests an increased risk for recurrence". Transplantation. 90 (11): 1243–1244. doi:10.1097/TP.0b013e3181f9caaa. PMID 21119507.
- "Routine HPV Testing in Head and Neck Squamous Cell Carcinoma. EBS 5-9". May 2013. Archived from the original on 30 September 2016. Retrieved 22 May 2017.
- Haines III GK (24 May 2013). "Pathology of Head and Neck Cancers I: Epithelial and Related Tumors". In Radosevich JA (ed.). Head & Neck Cancer: Current Perspectives, Advances, and Challenges. Springer Science & Business Media. pp. 257–87. ISBN 978-94-007-5827-8. Archived from the original on 7 January 2016.
- Wang YX, Hu D, Yan X (September 2013). "Diagnostic accuracy of Cyfra 21-1 for head and neck squamous cell carcinoma: a meta-analysis". European Review for Medical and Pharmacological Sciences. 17 (17): 2383–2389. PMID 24065233.
- Al-Sarraf M (2002). "Treatment of locally advanced head and neck cancer: historical and critical review". Cancer Control. 9 (5): 387–399. doi:10.1177/107327480200900504. PMID 12410178.
- Maxwell JH, Thompson LD, Brandwein-Gensler MS, Weiss BG, Canis M, Purgina B, et al. (December 2015). "Early Oral Tongue Squamous Cell Carcinoma: Sampling of Margins From Tumor Bed and Worse Local Control". JAMA Otolaryngology–Head & Neck Surgery. 141 (12): 1104–1110. doi:10.1001/jamaoto.2015.1351. PMC 5242089. PMID 26225798.
- Archived March 5, 2012, at the Wayback Machine
- Mahmood SS, Nohria A (July 2016). "Cardiovascular Complications of Cranial and Neck Radiation". Current Treatment Options in Cardiovascular Medicine. 18 (7): 45. doi:10.1007/s11936-016-0468-4. PMID 27181400. S2CID 23888595.
- Yan J, Yuan L, Wang J, Li S, Yao M, Wang K, Herst PM (September 2020). "Mepitel Film is superior to Biafine cream in managing acute radiation-induced skin reactions in head and neck cancer patients: a randomised intra-patient controlled clinical trial". Journal of Medical Radiation Sciences. 67 (3): 208–216. doi:10.1002/jmrs.397. PMC 7476193. PMID 32475079.
- "FDA Approval for Docetaxel - National Cancer Institute". Cancer.gov. Archived from the original on 2014-09-01. Retrieved 2014-08-07.
- Lambin P, Ramaekers BL, van Mastrigt GA, Van den Ende P, de Jong J, De Ruysscher DK, Pijls-Johannesma M (July 2009). "Erythropoietin as an adjuvant treatment with (chemo) radiation therapy for head and neck cancer". The Cochrane Database of Systematic Reviews (3): CD006158. doi:10.1002/14651858.CD006158.pub2. PMID 19588382.
- "Inoperable cancers killed by new laser surgery" The Times. UK. 3-April-2010 p15
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. (February 2006). "Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck". The New England Journal of Medicine. 354 (6): 567–578. doi:10.1056/NEJMoa053422. PMID 16467544. S2CID 8521075.
- Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA (December 2005). "Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study". Journal of Clinical Oncology. 23 (34): 8646–8654. doi:10.1200/JCO.2005.02.4646. PMID 16314626.
- Specenier P, Vermorken JB (May 2010). "Advances in the systemic treatment of head and neck cancers". Current Opinion in Oncology. 22 (3): 200–205. doi:10.1097/CCO.0b013e3283376e15. PMID 20154619. S2CID 20751568.
- Pearson S, Jia H, Kandachi K (January 2004). "China approves first gene therapy". Nature Biotechnology. 22 (1): 3–4. doi:10.1038/nbt0104-3. PMC 7097065. PMID 14704685.
- Lechner M, Frampton GM, Fenton T, Feber A, Palmer G, Jay A, et al. (2013). "Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV- tumors". Genome Medicine. 5 (5): 49. doi:10.1186/gm453. PMC 4064312. PMID 23718828.
- Center for Drug Evaluation and Research (2019-02-09). "pembrolizumab (KEYTRUDA)". FDA.
- Center for Drug Evaluation and Research (2018-11-03). "Nivolumab for SCCHN". FDA.
- Center for Drug Evaluation and Research (2019-06-11). "FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma". FDA.
- Semple C, Parahoo K, Norman A, McCaughan E, Humphris G, Mills M (July 2013). "Psychosocial interventions for patients with head and neck cancer". The Cochrane Database of Systematic Reviews (7): CD009441. doi:10.1002/14651858.CD009441.pub2. hdl:10026.1/3146. PMID 23857592. S2CID 42090352.
- Gourin CG, Podolsky RH (July 2006). "Racial disparities in patients with head and neck squamous cell carcinoma". The Laryngoscope. 116 (7): 1093–1106. doi:10.1097/01.mlg.0000224939.61503.83. PMID 16826042. S2CID 11140152.
- Iro H, Waldfahrer F (November 1998). "Evaluation of the newly updated TNM classification of head and neck carcinoma with data from 3247 patients". Cancer. 83 (10): 2201–2207. doi:10.1002/(SICI)1097-0142(19981115)83:10<2201::AID-CNCR20>3.0.CO;2-7. PMID 9827726.
- Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR (March 1995). "Second primary tumors in patients with head and neck squamous cell carcinoma". Cancer. 75 (6): 1343–1353. doi:10.1002/1097-0142(19950315)75:6<1343::AID-CNCR2820750617>3.0.CO;2-T. PMID 7882285.
- Cooper JS, Pajak TF, Rubin P, Tupchong L, Brady LW, Leibel SA, et al. (September 1989). "Second malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience". International Journal of Radiation Oncology, Biology, Physics. 17 (3): 449–456. doi:10.1016/0360-3016(89)90094-1. PMID 2674073.
- Priante AV, Castilho EC, Kowalski LP (April 2011). "Second primary tumors in patients with head and neck cancer". Current Oncology Reports. 13 (2): 132–137. doi:10.1007/s11912-010-0147-7. PMID 21234721. S2CID 207335139.
- Nugent B, Lewis S, O'Sullivan JM (January 2013). "Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy". The Cochrane Database of Systematic Reviews (1): CD007904. doi:10.1002/14651858.CD007904.pub3. PMC 6769131. PMID 23440820.
- Longacre ML, Ridge JA, Burtness BA, Galloway TJ, Fang CY (January 2012). "Psychological functioning of caregivers for head and neck cancer patients". Oral Oncology. 48 (1): 18–25. doi:10.1016/j.oraloncology.2011.11.012. PMC 3357183. PMID 22154127.
- Sherrod AM, Murphy BA, Wells NL, Bond SM, Hertzog M, Gilbert J, et al. (2014-05-20). "Caregiving burden in head and neck cancer". Journal of Clinical Oncology. 32 (15_suppl): e20678. doi:10.1200/jco.2014.32.15_suppl.e20678. ISSN 0732-183X.
- "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 2009-11-11. Retrieved Nov 11, 2009.
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006). "Cancer statistics, 2006". CA: A Cancer Journal for Clinicians. 56 (2): 106–130. doi:10.3322/canjclin.56.2.106. PMID 16514137. S2CID 21618776.
- Titcomb CP (2001). "High incidence of nasopharyngeal carcinoma in Asia". Journal of Insurance Medicine. 33 (3): 235–238. PMID 11558403.
- Cancer Facts and Figures, Archived 2007-09-29 at the Wayback Machine, American Cancer Society 2002.
- "Throat Cancer". Patient information web page. NCH Healthcare Systems. 1999. Archived from the original on 2007-07-01. Retrieved 2007-06-17.
- Reducing the Health Consequences of Smoking: 25 Years of Progress. A Report of the Surgeon General, U. S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, 1989.
- Syn NL, Teng MW, Mok TS, Soo RA (December 2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet. Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
External links
- Head and Neck Cancer at MedlinePlus (National Library of Medicine)
- Head and Neck Cancer Treatment at RadiologyInfo
- Head and Neck Cancer at Cancer.net (American Society of Clinical Oncology)