Protist locomotion
Protists are the eukaryotes that cannot be classified as plants, fungi or animals. They are mostly unicellular and microscopic. Many unicellular protists, particularly protozoans, are motile and can generate movement using flagella, cilia or pseudopods. Cells which use flagella for movement are usually referred to as flagellates, cells which use cilia are usually referred to as ciliates, and cells which use pseudopods are usually referred to as amoeba or amoeboids. Other protists are not motile, and consequently have no built-in movement mechanism.
| Part of a series on |
| Microbial and microbot movement |
|---|
.png.webp) |
| Microswimmers |
| Molecular motors |
|
Overview
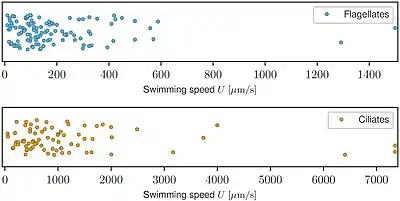
Unicellular protists comprise a vast, diverse group of organisms that covers virtually all environments and habitats, displaying a menagerie of shapes and forms. Hundreds of species of the ciliate genus Paramecium [3] or flagellated Euglena [4] are found in marine, brackish, and freshwater reservoirs; the green algae Chlamydomonas is distributed in soil and fresh water world-wide;[5] parasites from the genus Giardia colonize intestines of several vertebrate species.[6] One of the shared features of these organisms is their motility, crucial for nutrient acquisition and avoidance of danger.[7] In the process of evolution, single-celled organisms have developed in a variety of directions, and thus their rich morphology results in a large spectrum of swimming modes.[8][2]
Many swimming protists actuate tail-like appendages called flagella or cilia in order to generate the required thrust.[9] This is achieved by actively generating deformations along the flagellum, giving rise to a complex waveform. The flagellar axoneme itself is a bundle of nine pairs of microtubule doublets surrounding two central microtubules, termed the 9+2 axoneme,[10] and cross-linking dynein motors, powered by ATP hydrolysis, perform mechanical work by promoting the relative sliding of filaments, resulting in bending deformations.[2]
Although protist flagella have a diversity of forms and functions,[11] two large families, flagellates and ciliates, can be distinguished by the shape and beating pattern of their flagella.[2]
In the phylogenetic tree on the right, aquatic organisms (living in marine, brackish, or freshwater environments) have their branches drawn in blue while parasitic organisms have their branches drawn in red. Ciliates are indicated by an asterisk after their names. For each phylum marked in bold font, a representative organism has been sketched next to its name.[2]
Modes of locomotion
Protists according to how they move | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type of protist | Movement mechanism | Description | Example | Other examples | ||||
| Motile | Flagellates | 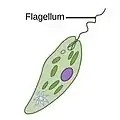 |
A flagellum (Latin for whip) is a lash-like appendage that protrudes from the cell body of some protists (as well as some bacteria). Flagellates use from one to several flagella for locomotion and sometimes as feeding and sensory organelle. |  |
Cryptophytes | All dinoflagellates and nanoflagellates (choanoflagellates, silicoflagellates, most green algae)[12][13] (Other protists go through a phase as gametes when they have temporary flagellum – some radiolarians, foraminiferans and Apicomplexa) | ||
| Ciliates | 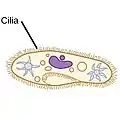 |
A cilium (Latin for eyelash) is a tiny flagellum. Ciliates use multiple cilia, which can number in many hundreds, to power themselves through the water. | .jpg.webp) |
Paramecium bursaria click to see cilia |
Foraminiferans, and some marine amoebae, ciliates and flagellates. | |||
| Amoebas (amoeboids) |
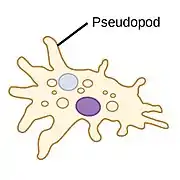 |
Pseudopods (Greek for false feet) are lobe-like appendages which amoebas use to anchor to a solid surface and pull themselves forward. They can change their shape by extending and retracting these pseudopods.[14] |  |
Amoeba | Found in every major protist lineage. Amoeboid cells occur among the protozoans, but also in the algae and the fungi.[15][16] | |||
| Not motile | none |
 |
Diatom | Diatoms, coccolithophores, and non‐motile species of Phaeocystis[13] Among protozoans the parasitic Apicomplexa are non‐motile. | ||||
Flagellates

Flagella are used in prokaryotes (archaea and bacteria) as well as protists. In addition, both flagella and cilia are widely used in eukaryotic cells (plant and animal) apart from protists.
The regular beat patterns of eukaryotic cilia and flagella generates motion on a cellular level. Examples range from the propulsion of single cells such as the swimming of spermatozoa to the transport of fluid along a stationary layer of cells such as in a respiratory tract. Though eukaryotic flagella and motile cilia are ultrastructurally identical, the beating pattern of the two organelles can be different. In the case of flagella, the motion is often planar and wave-like, whereas the motile cilia often perform a more complicated three-dimensional motion with a power and recovery stroke.
Eukaryotic flagella—those of animal, plant, and protist cells—are complex cellular projections that lash back and forth. Eukaryotic flagella are classed along with eukaryotic motile cilia as undulipodia[17] to emphasize their distinctive wavy appendage role in cellular function or motility. Primary cilia are immotile, and are not undulipodia.
Cryptaulax, Abollifer, Bodo, Rhynchomonas, Kittoksia, Allas, and Metromonas [18]
.jpg.webp) Freshwater green algal flagellate (Chlamydomonas)
Freshwater green algal flagellate (Chlamydomonas)
Flagellates typically have a small number of long flagella distributed along the bodies, and they actuate them to generate thrust. The set of observed movement sequences includes planar undulatory waves and traveling helical waves, either from the base to the tip, or in the opposite direction.[19][20] Flagella attached to the same body might follow different beating patterns, leading to a complex locomotion strategy that often relies also on the resistance the cell body poses to the fluid.[2]
Ciliates

In contrast to flagellates, propulsion of ciliates derives from the motion of a layer of densely-packed and collectively-moving cilia, which are short hair-like flagella covering their bodies. The seminal review paper of Brennen and Winet (1977) lists a few examples from both groups, highlighting their shape, beat form, geometric characteristics and swimming properties.[20] Cilia may also be used for transport of the surrounding fluid, and their cooperativity can lead to directed flow generation. In higher organisms this can be crucial for internal transport processes, as in cytoplasmic streaming within plant cells,[21] or the transport of ova from the ovary to the uterus in female mammals.[22][2]
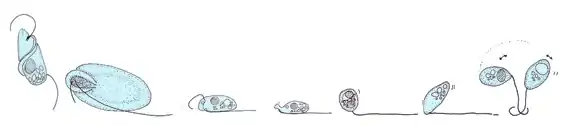
Ciliates generally have hundreds to thousands of cilia that are densely packed together in arrays. Like the flagella, the cilia are powered by specialised molecular motors. An efficient forward stroke is made with a stiffened flagellum, followed by an inefficient backward stroke made with a relaxed flagellum. During movement, an individual cilium deforms as it uses the high-friction power strokes and the low-friction recovery strokes. Since there are multiple cilia packed together on an individual organism, they display collective behaviour in a metachronal rhythm. This means the deformation of one cilium is in phase with the deformation of its neighbor, causing deformation waves that propagate along the surface of the organism. These propagating waves of cilia are what allow the organism to use the cilia in a coordinated manner to move. A typical example of a ciliated microorganism is the Paramecium, a one-celled, ciliated protozoan covered by thousands of cilia. The cilia beating together allow the Paramecium to propel through the water at speeds of 500 micrometers per second.[23]

 Paramecium feeding on bacteria
Paramecium feeding on bacteria The ciliate Oxytricha trifallax with cilia clearly visible
The ciliate Oxytricha trifallax with cilia clearly visible- Animation of swimming ciliate [24]
| External video | |
|---|---|
Amoebas


The third prevalent forms of protist cell motility is actin-dependent cell migration. The evolution of flagellar-based swimming has been well studied, and strong evidence suggests a single evolutionary origin for the eukaryotic flagellum occurred before the diversification of modern eukaryotes. On the other hand, actin-dependent crawling uses many different molecular mechanisms, and the study of how these evolved is only just beginning.[25]
Colonial protists

(b) Schematic of a colony of radius a: sixteen cells (green) each with one eye spot (orange dot). The cis flagellum is closest to the eye spot, the trans flagellum is furthest.[27] Flagella of the central cells beat in an opposing breaststroke, while the peripheral flagella beat in parallel. The pinwheel organization of the peripheral flagella leads to a left-handed body rotation at a rate ω3.
Gonium is a genus of colonial algae belonging to the family Volvocaceae. Typical colonies have 4 to 16 cells, all the same size, arranged in a flat plate, with no anterior-posterior differentiation. In a colony of 16 cells, four are in the center, and the other 12 are on the four sides, three each.[28]
Since the work of August Weismann on germ-plasm theory in biology [29] and of Julian Huxley on the nature of the individual in evolutionary theory,[30] the various species of green algae belonging to the family Volvocaceae have been recognized as important ones in the study of evolutionary transitions from uni- to multicellular life. In a modern biological view,[31] this significance arises from a number of specific features of these algae, including the fact that they are an extant family (obviating the need to study microfossils), are readily obtainable in nature, have been studied from a variety of perspectives (biochemical, developmental, genetic), and have had significant ecological studies. From a fluid dynamical perspective,[32] their relatively large size and easy culturing conditions allow for precise studies of their motility, the flows they create with their flagella, and interactions between organisms, while their high degree of symmetry simplifies theoretical descriptions of those same phenomena.[33][26]
As they are photosynthetic, the ability of these algae to execute phototaxis is central to their life. Because the lineage spans from unicellular to large colonial forms, it can be used to study the evolution of multicellular coordination of motility. Motility and phototaxis of motile green algae have been the subjects of an extensive literature in recent years,[34][35][36][37][38][39][40] focusing primarily on the two extreme cases: unicellular Chlamydomonas and much larger Volvox, with species composed of 1000–50,000 cells. Chlamydomonas swims typically by actuation of its two flagella in a breast stroke, combining propulsion and slow body rotation. It possesses an eyespot, a small area highly sensitive to light,[41][42] which triggers the two flagella differently.[43] Those responses are adaptive, on a timescale matched to the rotational period of the cell body,[44][45][46] and allow cells to scan the environment and swim toward light.[47] Multicellular Volvox shows a higher level of complexity, with differentiation between interior germ cells and somatic cells dedicated to propulsion. Despite lacking a central nervous system to coordinate its cells, Volvox exhibits accurate phototaxis. This is also achieved by an adaptive response to changing light levels, with a response time tuned to the colony rotation period which creates a differential response between the light and dark sides of the spheroid.[35][48][26]
In light of the above, a natural questions is as follows: How does the simplest differentiated organism achieve phototaxis? In the Volvocine lineage the species of interest is Gonium. This 8- or 16-cell colony represents one of the first steps to true multicellularity,[49] presumed to have evolved from the unicellular common ancestor earlier than other Volvocine algae.[50] It is also the first to show cell differentiation.[26]
The 16-cell Gonium colony shown in the diagram on the right is organized into two concentric squares of respectively 4 and 12 cells, each biflagellated, held together by an extracellular matrix.[51] All flagella point out on the same side: It exhibits a much lower symmetry than Volvox, lacking anterior-posterior symmetry. Yet it performs similar functions to its unicellular and large colonies counterparts as it mixes propulsion and body rotation and swims efficiently toward light.[34][52][53] The flagellar organization of inner and peripheral cells deeply differs:[54][55] Central cells are similar to Chlamydomonas, with the two flagella beating in an opposing breast stroke, and contribute mostly to the forward propulsion of the colony. Cells at the periphery, however, have flagella beating in parallel, in a fashion close to Volvox cells.[27] This minimizes steric interactions and avoids flagella crossing each other.[34] Moreover, these flagella are implanted with a slight angle and organized in a pinwheel fashion [see Fig. 1(b)]:[54] Their beating induces a left-handed rotation of the colony, highlighted in Figs. 1(c) and 1(d) and in Supplemental Movie 1 [29]. Therefore, the flagella structure of Gonium reinforces its key position as intermediate in the evolution toward multicellularity and cell differentiation.[26]
These small flat assemblies show intriguing swimming along helical trajectories—with their body plane almost normal to the swimming direction—that have attracted the attention of naturalists since the 18th century.[52][53][56] Yet the way in which Gonium colonies bias their swimming toward the light remains unclear. Early microscopic observations have identified differential flagellar activity between the illuminated and the shaded sides of the colony as the source of phototactic reorientation.[52][53] Yet a full fluid-dynamics description, quantitatively linking the flagellar response to light variations and the hydrodynamic forces and torques acting on the colony, is still lacking. From an evolutionary perspective, phototaxis in Gonium raises fundamental issues such as the extent to which the phototactic strategy of the unicellular ancestor is retained in the colonial form, how the phototactic flagella reaction adapted to the geometry and symmetry of the colony, and how it leads to effective reorientation.[26]
Protist taxis: Directed motion
Phototaxis
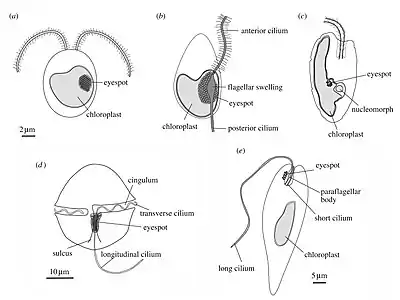
(a) green alga (b) heterokont zoospore (c) cryptomonad alga
(d) dinoflagellate (e) Euglena
Some protists can move toward or away from a stimulus, a movement referred to as taxis. For example, movement toward light, termed phototaxis, is accomplished by coupling their locomotion strategy with a light-sensing organ.[58] Eukaryotes evolved for the first time in the history of life the ability to follow light direction in three dimensions in open water. The strategy of eukaryotic sensory integration, sensory processing and the speed and mechanics of tactic responses is fundamentally different from that found in prokaryotes.[59][57]
Both single-celled and multi-cellular eukaryotic phototactic organisms have a fixed shape, are polarized, swim in a spiral and use cilia for swimming and phototactic steering. Signalling can happen via direct light-triggered ion currents, adenylyl cyclases or trimeric G-proteins. The photoreceptors used can also be very different (see below). However, signalling in all cases eventually modifies the beating activity of cilia.[57] The mechanics of phototactic orientation is analogous in all eukaryotes. A photosensor with a restricted view angle rotates to scan the space and signals periodically to the cilia to alter their beating, which will change the direction of the helical swimming trajectory. Three-dimensional phototaxis can be found in five out of the six eukaryotic major groups (opisthokonts, Amoebozoa, plants, chromalveolates, excavates, rhizaria).[57]
Pelagic phototaxis is present in green algae – it is not present in glaucophyte algae or red algae.[57] Green algae have a "stigma" located in the outermost portion of the chloroplast, directly underneath the two chloroplast membranes. The stigma is made of tens to several hundreds of lipid globules, which often form hexagonal arrays and can be arranged in one or more rows. The lipid globules contain a complex mixture of carotenoid pigments, which provide the screening function and the orange-red colour,[60] as well as proteins that stabilize the globules.[61] The stigma is located laterally, in a fixed plane relative to the cilia, but not directly adjacent to the basal bodies.[62][63] The fixed position is ensured by the attachment of the chloroplast to one of the ciliary roots.[64] The pigmented stigma is not to be confused with the photoreceptor. The stigma only provides directional shading for the adjacent membrane-inserted photoreceptors (the term "eyespot" is therefore misleading). Stigmata can also reflect and focus light like a concave mirror, thereby enhancing sensitivity.[57]
In the best-studied green alga, Chlamydomonas reinhardtii, phototaxis is mediated by a rhodopsin pigment, as first demonstrated by the restoration of normal photobehaviour in a blind mutant by analogues of the retinal chromophore.[65] Two archaebacterial-type rhodopsins, channelrhodopsin-1 and -2,[66][67] were identified as phototaxis receptors in Chlamydomonas.[68] Both proteins have an N-terminal 7-transmembrane portion, similar to archaebacterial rhodopsins, followed by an approximately 400 residue C-terminal membrane-associated portion. CSRA and CSRB act as light-gated cation channels and trigger depolarizing photocurrents.[68][69] CSRA was shown to localize to the stigma region using immunofluorescence analysis (Suzuki et al. 2003). Individual RNAi depletion of both CSRA and CSRB modified the light-induced currents and revealed that CSRA mediates a fast, high-saturating current while CSRB a slow, low-saturating one. Both currents are able to trigger photophobic responses and can have a role in phototaxis,[70][69] although the exact contribution of the two receptors is not yet clear.[57]
As in all bikonts (plants, chromalveolates, excavates, rhizaria), green algae have two cilia, which are not identical. The anterior cilium is always younger than the posterior one.[71][72] In every cell cycle, one daughter cell receives the anterior cilium and transforms it into a posterior one. The other daughter inherits the posterior, mature cilium. Both daughters then grow a new anterior cilium.[57]
As all other ciliary swimmers, green algae always swim in a spiral. The handedness of the spiral is robust and is guaranteed by the chirality of the cilia. The two cilia of green algae have different beat patterns and functions. In Chlamydomonas, the phototransduction cascade alters the stroke pattern and beating speed of the two cilia differentially in a complex pattern.[44][45] This results in the reorientation of the helical swimming trajectory as long as the helical swimming axis is not aligned with the light vector.[57]
Thermotaxis
.jpg.webp)
Temperature is a key environmental factor for living organisms because chemical reaction rates and physical characteristics of biological materials can change substantially with temperature. Living organisms acclimate to cold and heat stress using acquired mechanisms, including the ability to migrate to an environment with temperatures suitable for inhabitation. One of the simplest forms of the behavior to migrate to a suitable thermal environment is thermotaxis. Thermotaxis has been found in multicellular organisms, such as Caenorhabditis elegans and Drosophila melanogaster, as well as in unicellular organisms, such as Paramecium caudatum, Dictyostelium discoideum, Physarum polycephalum, and Escherichia coli.[74] Individual cells within multicellular organisms also show thermotaxis. For example, mammalian sperm migrate through the oviduct to the fertilization site guided by a rise in temperature.[75][76]
The investigation of how unicellular organisms migrate toward preferred temperatures began more than 100 years ago.[74] In particular, the thermotactic behavior of Paramecium cells has been well studied. Paramecium cells accumulate at sites that are close to the cultivation temperature, i. e. the temperature at which cells are grown.[74] Accumulation at these sites occurs because cells frequently reverse their swimming direction when they encounter a temperature change that deviates from the cultivation temperature and increase their swimming velocity when they experience a temperature change that approaches the cultivation temperature.[77][78] The reversal in swimming direction is induced by a depolarizing receptor potential, which triggers an action potential in the cilia.[79] These studies on Paramecium cells highlighted the thermotaxis in unicellular organisms more than 30 years ago, but the molecular mechanisms for thermoreception and signal transduction are not yet understood.[76]
The understanding of the molecular mechanisms for thermotaxis has progressed greatly in recent years, from investigations of mammalian sperm. Human sperm migrates toward warmer temperatures, ranging from 29 °C to 41 °C.[75] Sperm can detect a temperature gradient as small as 0.014 °C/mm, suggesting that sperm detect temporal changes in temperature rather than spatial differences.[75] Several molecules have been proposed to be sensor molecules, including opsin and transient receptor potential (TRP) channels such as TRPV1, TRPV4, and TRPM8.[80][81][82] TRP channels are multimodal sensor for thermal, chemical and mechanical stimuli, but the function of opsins as a thermosensor awaits to be established.[76]
Temperature is a critical environmental factor also for Chlamydomonas cells, which produce small heat shock proteins, chaperonins, and HSP70 heat shock proteins, and also undergo other heat shock responses to cope with heat stress.[83][84][85][86] In response to a cold shock of 4 °C, cells halt proliferation and accumulate starch and sugar.[87] Behavioral responses to avoid stressful warm or cold environments are expected to be present in Chlamydomonas. Although C. moewusii cells are reported to migrate toward warmer temperatures in a 10 °C to 15 °C gradient,[88] there has been no report in which the temperature range was systematically manipulated to examine a relationship with cultivation temperature. A 2019 study demonstrated thermotaxis in Chlamydomonas reinhardtii, and found that between 10 °C and 30 °C Chlamydomonas cells migrated toward lower temperatures independent of cultivation temperature.[76]
In contrast to thermotaxis, phototaxis has been extensively studied in Chlamydomonas. Two flagella of Chlamydomonas beat in a breast-stroke like pattern during forward swimming and, during phototaxis, Chlamydomonas cells make by a turn toward or away from a light source by controlling the balance of the propulsive forces generated by the two flagella.[41][89] The balance depends on the intraflagellar calcium ion concentration; thus, loss of calcium-dependent control in ptx1 mutants results in a phototaxis defect.[43][90][91] The direction of phototaxis in Chlamydomonas depends on the light intensity, but is also affected by intracellular reduction-oxidation (redox) conditions.[92] Cells migrate toward a light source when the light intensity is weak, but the direction reverses under reducing conditions. In contrast, cells swim away from light sources with strong intensity, but the direction reverses under oxidizing conditions.[76]
Swimming speeds

Escape response: Action potentials
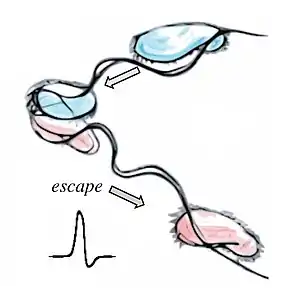
In flagellate algae, abrupt changes in light intensity or intense photic stimuli induce rapid flagellar reversal and transient backward swimming.[94][95] In green algae, this action may be mediated by the contractile root fibre which alters the angle between basal bodies.[96] Cells can also react at speed to unexpected mechanical stimuli. All-or-none contractions in the stalked ciliate Vorticella can occur at rates of 8 cm s−1.[97] In some species of heliozoa, axopods can completely retract within 20 ms in order to draw in trapped prey for phagocytosis.[97][98][93]
These fast reactions are usually induced by action potentials — unidirectional electrical pulses involving fast, regenerative changes in membrane potential. While all cells display some electrical activity, phylogenetic evidence suggests that the capacity to propagate action potentials may have been an ancestral eukaryotic trait supported by the last eukaryotic common ancestor. These may have emerged in response to accidental membrane damage and sudden calcium influx.[99] Bioelectrical signalling in the form of action potentials occurs orders of magnitude faster than any other signalling modalities, e.g. chemical diffusion, protein phosphorylation etc.[93]
In order to initiate fast escape responses, these may have been coupled directly to the motility apparatus—particularly to flexible, membrane-continuous structures such as cilia and pseudopodia. Loss of voltage-gated sodium/calcium channels is further correlated with loss of cilia in many taxa. In protists, all-or-none action potentials occur almost exclusively in association with ciliary membranes,[100][101][102] with the exception of some non-ciliated diatoms.[103][104] Graded potentials occur in amoebae, also for movement control.[105][93]

In Chlamydomonas, action-potential-like flagellar currents induce photophobic responses and flagella reversal (via the voltage-gated calcium channel Cav2), while photoreceptor currents elicit much milder responses.[106] Here, a mechanosensory channel of the transient receptor potential family is localized to the ciliary base, while Cav2 is localized only to the distal regions of cilia.[107][108] In Paramecium, hyperpolarizations increase ciliary beat frequency, while depolarizations have the opposite effect and eventually lead to a ciliary reversal. Depolarizations above a certain threshold result in action potentials, owing to opening of Cav channels located exclusively in the ciliary membrane.[109][110] Potassium channels — also residing in the membrane — help restore the resting membrane potential.[93]
Eukaryotes manipulate their membrane potential to achieve transitions between different behaviours. Complex bioelectric sequences have been recorded in association with integrated feeding and predation behaviours in Favella.[111] Repetitive behaviours arise from rhythmic spiking. In ciliates, rhythmic depolarizations control fast and slow walking by tentacle-like compound cilia called cirri,[112] enabling escape from dead ends [113] and courtship rituals in conjugating gametes.[114][115] In Stentor, action potentials produce whole-body contractions.[116] Finally, excitable systems operating close to bifurcations may admit limit cycles, which manifest as repetitive or rhythmic electrical spiking and repetitive behaviours. Ultimately, this may lead to habituation.[117][118][93]
Biohybrid microswimmers
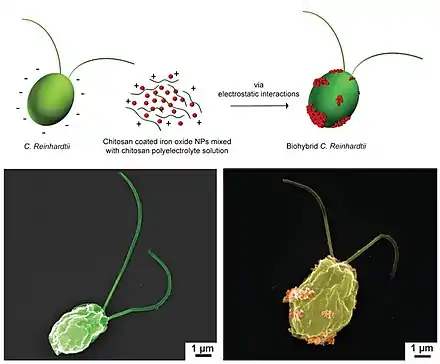
Bottom: SEM images of bare microalgae (left) and biohybrid microalgae (right) coated with chitosan-coated iron oxide nanoparticles (CSIONPs). Images were pseudocolored. A darker green color on the right SEM image represents chitosan coating on microalgae cell wall. Orange-colored particles represents CSIONPs.
Biohybrid microswimmers can be defined as microswimmers that consist of both biological and artificial constituents, for instance, one or several living microorganisms attached to one or various synthetic parts.[120][121] The pioneers of this field, ahead of their time, were Montemagno and Bachand with a 1999 work regarding specific attachment strategies of biological molecules to nanofabricated substrates enabling the preparation of hybrid inorganic/organic nanoelectromechanical systems, so called NEMS.[122] They described the production of large amounts of F1-ATPase from the thermophilic bacteria Bacillus PS3 for the preparation of F1-ATPase biomolecular motors immobilized on a nanoarray pattern of gold, copper or nickle produced by electron beam lithography. These proteins were attached to one micron microspheres tagged with a synthetic peptide. Consequently, they accomplished the preparation of a platform with chemically active sites and the development of biohybrid devices capable of converting energy of biomolecular motors into useful work.[121]
Over the past decade, biohybrid microrobots, in which living mobile microorganisms are physically integrated with untethered artificial structures, have gained growing interest to enable the active locomotion and cargo delivery to a target destination.[123][124][125][126] In addition to the motility, the intrinsic capabilities of sensing and eliciting an appropriate response to artificial and environmental changes make cell-based biohybrid microrobots appealing for transportation of cargo to the inaccessible cavities of the human body for local active delivery of diagnostic and therapeutic agents.[127][128][129] Active locomotion, targeting and steering of concentrated therapeutic and diagnostic agents embedded in mobile microrobots to the site of action can overcome the existing challenges of conventional therapies.[130][131][132] To this end, bacteria have been commonly used with attached beads and ghost cell bodies.[133][134][135][136][137][138][139][140][119]
Chlamydomonas reinhardtii is a unicellular green microalga. The wild-type C. reinhardtii has a spherical shape that averages about 10 µm in diameter.[141] This microorganism can perceive the visible light and be steered by it (i.e., phototaxis) with high swimming speeds in the range of 100–200 µm s−1.[129] It has natural autofluorescence that permits label-free fluorescent imaging.[141] C. reinhardtii has been actively explored as the live component of biohybrid microrobots for the active delivery of therapeutics.[129] They are biocompatible with healthy mammalian cells, leave no known toxins, mobile in the physiologically relevant media, and allow for surface modification to carry cargo on the cell wall.[129][142][143][144][145] Alternative attachment strategies for C. reinhardtii have been proposed for the assembly through modifying the interacting surfaces by electrostatic interactions[129][142] and covalent bonding.[146] [119]
See also
- Aquatic locomotion
- Bacterial motility
- Cytoskeleton
- Spirostomum
- Squirmer
- Vorticella
References
- Hinchliff, Cody E.; Smith, Stephen A.; Allman, James F.; Burleigh, J. Gordon; Chaudhary, Ruchi; Coghill, Lyndon M.; Crandall, Keith A.; Deng, Jiabin; Drew, Bryan T.; Gazis, Romina; Gude, Karl; Hibbett, David S.; Katz, Laura A.; Laughinghouse, H. Dail; McTavish, Emily Jane; Midford, Peter E.; Owen, Christopher L.; Ree, Richard H.; Rees, Jonathan A.; Soltis, Douglas E.; Williams, Tiffani; Cranston, Karen A. (2015). "Synthesis of phylogeny and taxonomy into a comprehensive tree of life". Proceedings of the National Academy of Sciences. 112 (41): 12764–12769. Bibcode:2015PNAS..11212764H. doi:10.1073/pnas.1423041112. PMC 4611642. PMID 26385966.
- Lisicki, Maciej; Velho Rodrigues, Marcos F.; Goldstein, Raymond E.; Lauga, Eric (2019). "Swimming eukaryotic microorganisms exhibit a universal speed distribution". eLife. 8. arXiv:1907.00906. doi:10.7554/eLife.44907. PMC 6634970. PMID 31310238.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Wichterman, R. (6 December 2012). The Biology of Paramecium. ISBN 9781475703726.
- Buetow, Dennis E. (2011). "Euglena". eLS. doi:10.1002/9780470015902.a0001964.pub3. ISBN 978-0470016176.
- Harris, Elizabeth H. (7 March 2009). The Chlamydomonas Sourcebook: Introduction to Chlamydomonas and Its Laboratory Use: Volume 1. ISBN 97-80080919553.
- Adam, Rodney D. (2001). "Biology of Giardia lamblia". Clinical Microbiology Reviews. 14 (3): 447–475. doi:10.1128/CMR.14.3.447-475.2001. PMC 88984. PMID 11432808.
- Bray, Dennis (2 November 2000). Cell Movements: From Molecules to Motility. ISBN 9781136844355.
- Cappuccinelli, P. (1980). "The movement of eukaryotic cells". Motility of Living Cells. pp. 59–74. doi:10.1007/978-94-009-5812-8_4. ISBN 978-0-412-15770-7.
- Sleigh, M. A. (1974) Cilia and flagella. Academic Press.
- Nicastro, D.; McIntosh, J. R.; Baumeister, W. (2005). "3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography". Proceedings of the National Academy of Sciences. 102 (44): 15889–15894. Bibcode:2005PNAS..10215889N. doi:10.1073/pnas.0508274102. PMC 1276108. PMID 16246999.
- Moran, Jonathan; McKean, Paul G.; Ginger, Michael L. (2014). "Eukaryotic Flagella: Variations in Form, Function, and Composition during Evolution". BioScience. 64 (12): 1103–1114. doi:10.1093/biosci/biu175. ISSN 1525-3244.
- Dawson, Scott C; Paredez, Alexander R (2013). "Alternative cytoskeletal landscapes: cytoskeletal novelty and evolution in basal excavate protists". Current Opinion in Cell Biology. 25 (1): 134–141. doi:10.1016/j.ceb.2012.11.005. PMC 4927265. PMID 23312067.
- Atkinson, A.; Polimene, L.; Fileman, E.S.; Widdicombe, C.E.; McEvoy, A.J.; Smyth, T.J.; Djeghri, N.; Sailley, S.F.; Cornwell, L.E. (2018). ""Comment. What drives plankton seasonality in a stratifying shelf sea? Some competing and complementary theories"]" (PDF). Limnology and Oceanography. 63 (6): 2877–2884. Bibcode:2018LimOc..63.2877A. doi:10.1002/lno.11036. S2CID 91380765.
- Singleton, Paul (2006). Dictionary of Microbiology and Molecular Biology, 3rd Edition, revised. Chichester, UK: John Wiley & Sons. pp. 32. ISBN 978-0-470-03545-0.
- David J. Patterson. "Amoebae: Protists Which Move and Feed Using Pseudopodia". Tree of Life web project.
- "The Amoebae". The University of Edinburgh. Archived from the original on 10 June 2009.
- A Dictionary of Biology, 2004, accessed 2011-01-01.
- Patterson, David J. (2000) "Flagellates: Heterotrophic Protists With Flagella" Tree of Life.
- Jahn, T. L.; Votta, J. J. (1972). "Locomotion of Protozoa". Annual Review of Fluid Mechanics. 4: 93–116. Bibcode:1972AnRFM...4...93J. doi:10.1146/annurev.fl.04.010172.000521.
- Brennen, C.; Winet, H. (1977). "Fluid Mechanics of Propulsion by Cilia and Flagella". Annual Review of Fluid Mechanics. 9: 339–398. Bibcode:1977AnRFM...9..339B. doi:10.1146/annurev.fl.09.010177.002011.
- Allen, N. S.; Allen, R. D. (1978). "Cytoplasmic Streaming in Green Plants". Annual Review of Biophysics and Bioengineering. 7: 497–526. doi:10.1146/annurev.bb.07.060178.002433. PMID 352247.
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. (2006). "The reproductive significance of human Fallopian tube cilia". Human Reproduction Update. 12 (4): 363–372. doi:10.1093/humupd/dml012. PMID 16565155.
- Lauga, Eric; Thomas R Powers (25 August 2009). "The hydrodynamics of swimming microorganisms". Reports on Progress in Physics. 72 (9): 096601. arXiv:0812.2887. Bibcode:2009RPPh...72i6601L. doi:10.1088/0034-4885/72/9/096601. S2CID 3932471.
- Rode, Sebastian; Elgeti, Jens; Gompper, Gerhard (2021). "Multi-ciliated microswimmers–metachronal coordination and helical swimming". The European Physical Journal E. 44 (6): 76. arXiv:2103.11447. doi:10.1140/epje/s10189-021-00078-x. PMC 8187229. PMID 34101070.
- Fritz-Laylin, Lillian K. (2020). "The evolution of animal cell motility". Current Biology. 30 (10): R477–R482. doi:10.1016/j.cub.2020.03.026. PMID 32428485. S2CID 218711237.
- De Maleprade, Hélène; Moisy, Frédéric; Ishikawa, Takuji; Goldstein, Raymond E. (2020). "Motility and phototaxis of Gonium, the simplest differentiated colonial alga". Physical Review E. 101 (2): 022416. arXiv:1911.08837. Bibcode:2020PhRvE.101b2416D. doi:10.1103/PhysRevE.101.022416. PMID 32168596. S2CID 211858528.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Coleman, A. W. (2012). "A Comparative Analysis of the Volvocaceae (Chlorophyta)1". Journal of Phycology. 48 (3): 491–513. doi:10.1111/j.1529-8817.2012.01168.x. PMID 27011065. S2CID 422091.
- Pennak, Robert W (1978). Fresh-Water Invertebrates of the United States (Second ed.). John Wiley & Sons. pp. 43. ISBN 0-471-04249-8.
- Weismann, August (1889). "Essays Upon Heredity and Kindred Biological Problems: By Dr. August Weismann ... Ed. By Edward B. Poulton ... Selmar Schönland ... And Arthur e. Shipley...Authorised Translation".
- Huxley, Julian S. (22 March 2012). The Individual in the Animal Kingdom. ISBN 9781107606074.
- Kirk, David L. (8 September 2005). Volvox: A Search for the Molecular and Genetic Origins of Multicellularity and Cellular Differentiation. ISBN 9780521019149.
- Goldstein, Raymond E. (2015). "Green Algae as Model Organisms for Biological Fluid Dynamics". Annual Review of Fluid Mechanics. 47: 343–375. doi:10.1146/annurev-fluid-010313-141426. PMC 4650200. PMID 26594068.
- Goldstein, Raymond E. (2016). "Batchelor Prize Lecture Fluid dynamics at the scale of the cell". Journal of Fluid Mechanics. 807: 1–39. Bibcode:2016JFM...807....1G. doi:10.1017/jfm.2016.586. S2CID 55745525.
- Hoops, H. J. (1997). "Motility in the colonial and multicellular Volvocales: Structure, function, and evolution". Protoplasma. 199 (3–4): 99–112. doi:10.1007/BF01294499. S2CID 22315728.
- Drescher, K.; Goldstein, R. E.; Tuval, I. (2010). "Fidelity of adaptive phototaxis". Proceedings of the National Academy of Sciences. 107 (25): 11171–11176. doi:10.1073/pnas.1000901107. PMC 2895142. PMID 20534560.
- Drescher, Knut; Goldstein, Raymond E.; Michel, Nicolas; Polin, Marco; Tuval, Idan (2010). "Direct Measurement of the Flow Field around Swimming Microorganisms". Physical Review Letters. 105 (16): 168101. arXiv:1008.2681. Bibcode:2010PhRvL.105p8101D. doi:10.1103/PhysRevLett.105.168101. PMID 21231017. S2CID 8306079.
- Guasto, Jeffrey S.; Johnson, Karl A.; Gollub, J. P. (2010). "Oscillatory Flows Induced by Microorganisms Swimming in Two Dimensions". Physical Review Letters. 105 (16): 168102. arXiv:1008.2535. Bibcode:2010PhRvL.105p8102G. doi:10.1103/PhysRevLett.105.168102. PMID 21231018. S2CID 9533722.
- Bennett, Rachel R.; Golestanian, Ramin (2015). "A steering mechanism for phototaxis in Chlamydomonas". Journal of the Royal Society Interface. 12 (104). doi:10.1098/rsif.2014.1164. PMC 4345482. PMID 25589576.
- Arrieta, Jorge; Barreira, Ana; Chioccioli, Maurizio; Polin, Marco; Tuval, Idan (2017). "Phototaxis beyond turning: Persistent accumulation and response acclimation of the microalga Chlamydomonas reinhardtii". Scientific Reports. 7 (1): 3447. arXiv:1611.08224. Bibcode:2017NatSR...7.3447A. doi:10.1038/s41598-017-03618-8. PMC 5471259. PMID 28615673.
- Tsang, Alan C. H.; Lam, Amy T.; Riedel-Kruse, Ingmar H. (2018). "Polygonal motion and adaptable phototaxis via flagellar beat switching in the microswimmer Euglena gracilis". Nature Physics. 14 (12): 1216–1222. Bibcode:2018NatPh..14.1216T. doi:10.1038/s41567-018-0277-7. S2CID 126294173.
- Foster, K. W.; Smyth, R. D. (1980). "Light Antennas in phototactic algae". Microbiological Reviews. 44 (4): 572–630. doi:10.1128/mr.44.4.572-630.1980. PMC 373196. PMID 7010112.
- Hegemann, Peter (2008). "Algal Sensory Photoreceptors". Annual Review of Plant Biology. 59: 167–189. doi:10.1146/annurev.arplant.59.032607.092847. PMID 18444900.
- Kamiya, R.; Witman, G. B. (1984). "Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas". Journal of Cell Biology. 98 (1): 97–107. doi:10.1083/jcb.98.1.97. PMC 2112995. PMID 6707098.
- Josef, Keith; Saranak, Jureepan; Foster, Kenneth W. (2005). "Ciliary behavior of a negatively phototactic Chlamydomonas reinhardtii". Cell Motility and the Cytoskeleton. 61 (2): 97–111. doi:10.1002/cm.20069. PMID 15849714.
- Josef, Keith; Saranak, Jureepan; Foster, Kenneth W. (2006). "Linear systems analysis of the ciliary steering behavior associated with negative-phototaxis in Chlamydomonas reinhardtii". Cell Motility and the Cytoskeleton. 63 (12): 758–777. doi:10.1002/cm.20158. PMID 16986140.
- Yoshimura, Kenjiro; Kamiya, Ritsu (2001). "The Sensitivity of Chlamydomonas Photoreceptor is Optimized for the Frequency of Cell Body Rotation". Plant and Cell Physiology. 42 (6): 665–672. doi:10.1093/pcp/pce084. PMID 11427687.
- Leptos, Kyriacos C.; Chioccioli, Maurizio; Furlan, Silvano; Pesci, Adriana I.; Goldstein, Raymond E. (2018). "An Adaptive Flagellar Photoresponse Determines the Dynamics of Accurate Phototactic Steering in Chlamydomonas". doi:10.1101/254714. S2CID 90374721.
{{cite journal}}: Cite journal requires|journal=(help) - Kirk, David L. (2004). "Volvox". Current Biology. 14 (15): R599–R600. doi:10.1016/j.cub.2004.07.034. PMID 15296767. S2CID 235312006.
- Arakaki, Yoko; Kawai-Toyooka, Hiroko; Hamamura, Yuki; Higashiyama, Tetsuya; Noga, Akira; Hirono, Masafumi; Olson, Bradley J. S. C.; Nozaki, Hisayoshi (2013). "The Simplest Integrated Multicellular Organism Unveiled". PLOS ONE. 8 (12): e81641. Bibcode:2013PLoSO...881641A. doi:10.1371/journal.pone.0081641. PMC 3859500. PMID 24349103.
- Herron, Matthew D.; Michod, Richard E. (2008). "Evolution of Complexity in the Volvocine Algae: Transitions in Individuality Through Darwin's Eye". Evolution. 62 (2): 436–451. doi:10.1111/j.1558-5646.2007.00304.x. PMID 18031303. S2CID 12139760.
- Nozaki, Hisayoshi (1990). "Ultrastructure of the extracellular matrix of Gonium (Volvocales, Chlorophyta)". Phycologia. 29: 1–8. doi:10.2216/i0031-8884-29-1-1.1.
- Moore, A. R. (1916). "The mechanism of orientation in Gonium". Journal of Experimental Zoology. 21 (3): 431–432. doi:10.1002/jez.1400210306.
- Mast, S. O. (1916). "The process of orientation in the colonial organism, Gonium pectorale, and a study of the structure and function of the eye-spot". Journal of Experimental Zoology. 20: 1–17. doi:10.1002/jez.1400200102.
- Greuel, Brian T.; Floyd, Gary L. (1985). "Development of the Flagellar Apparatus and Flagellar Orientation in the Colonial Green Alga Gonium Pectorale (Volvocales)1". Journal of Phycology. 21 (3): 358–371. doi:10.1111/j.0022-3646.1985.00358.x. S2CID 85760904.
- Harper, R. A. (1912). "The Structure and Development of the Colony in Gonium". Transactions of the American Microscopical Society. 31 (2): 65–83. doi:10.2307/3221328. JSTOR 3221328.
- Müller O. F. (1782) Kleine Schriften Aus Der Naturhistorie, Dessau, herausgegeben von JAE Goeze, pp. 15–21.
- Jékely, Gáspár (2009). "Evolution of phototaxis". Philosophical Transactions of the Royal Society B: Biological Sciences. 364 (1531): 2795–2808. doi:10.1098/rstb.2009.0072. PMC 2781859. PMID 19720645.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Clark, M.A., Choi, J. and Douglas, M. (2018) Characteristics of Protists Biology 2e. OpenStax. ISBN 9781947172951.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - Häder, D. -P; Lebert, M. (19 June 2001). Photomovement. ISBN 9780080538860.
- Grung, Merete; Kreimer, Georg; Calenberg, Michael; Melkonian, Michael; Liaaen-Jensen, Synnøve (1994). "Carotenoids in the eyespot apparatus of the flagellate green alga Spermatozopsis similis: Adaptation to the retinal-based photoreceptor". Planta. 193. doi:10.1007/BF00191604. S2CID 29443649.
- Renninger, S.; Backendorf, E.; Kreimer, G. (2001). "Subfractionation of eyespot apparatuses from the green alga Spermatozopsis similis : Isolation and characterization of eyespot globules". Planta. 213 (1): 51–63. doi:10.1007/s004250000473. PMID 11523656. S2CID 24880210.
- Arnott, Howard J.; Brown, R. Malcolm (1967). "Ultrastructure of the Eyespot and its Possible Significance in Phototaxis of Tetracystis excentrica*†". The Journal of Protozoology. 14 (4): 529–539. doi:10.1111/j.1550-7408.1967.tb02038.x.
- Melkonian, M.; Robenek, H. (1979). "The eyespot of the flagellate Tetraselmis cordiformis stein (Chlorophyceae): Structural spezialization of the outer chloroplast membrane and its possible significance in phototaxis of green algae". Protoplasma. 100 (2): 183–197. doi:10.1007/BF01283929. S2CID 24606055.
- Melkonian, Michael (1978). "Structure and significance of cruciate flagellar root systems in green algae: Comparative investigations in species of Chlorosarcinopsis (Chlorosarcinales)". Plant Systematics and Evolution. 130 (3–4): 265–292. doi:10.1007/BF00982810. S2CID 22938771.
- Foster, Kenneth W.; Saranak, Jureepan; Patel, Nayana; Zarilli, Gerald; Okabe, Masami; Kline, Toni; Nakanishi, Koji (1984). "A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas". Nature. 311 (5988): 756–759. Bibcode:1984Natur.311..756F. doi:10.1038/311756a0. PMID 6493336. S2CID 4263301.
- Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Musti, A. M.; Bamberg, E.; Hegemann, P. (2002). "Channelrhodopsin-1: A Light-Gated Proton Channel in Green Algae". Science. 296 (5577): 2395–2398. Bibcode:2002Sci...296.2395N. doi:10.1126/science.1072068. PMID 12089443. S2CID 206506942.
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. (2003). "Channelrhodopsin-2, a directly light-gated cation-selective membrane channel". Proceedings of the National Academy of Sciences. 100 (24): 13940–13945. Bibcode:2003PNAS..10013940N. doi:10.1073/pnas.1936192100. PMC 283525. PMID 14615590.
- Sineshchekov, O. A.; Jung, K.-H.; Spudich, J. L. (2002). "Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii". Proceedings of the National Academy of Sciences. 99 (13): 8689–8694. doi:10.1073/pnas.122243399. PMC 124360. PMID 12060707.
- Berthold, Peter; Tsunoda, Satoshi P.; Ernst, Oliver P.; Mages, Wolfgang; Gradmann, Dietrich; Hegemann, Peter (2008). "Channelrhodopsin-1 Initiates Phototaxis and Photophobic Responses in Chlamydomonas by Immediate Light-Induced Depolarization". The Plant Cell. 20 (6): 1665–1677. doi:10.1105/tpc.108.057919. PMC 2483371. PMID 18552201.
- Govorunova, Elena G.; Jung, Kwang-Hwan; Sineshchekov, Oleg A.; Spudich, John L. (2004). "Chlamydomonas Sensory Rhodopsins a and B: Cellular Content and Role in Photophobic Responses". Biophysical Journal. 86 (4): 2342–2349. Bibcode:2004BpJ....86.2342G. doi:10.1016/S0006-3495(04)74291-5. PMC 1304083. PMID 15041672.
- Cavalier-Smith, T. (2002). "The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa". International Journal of Systematic and Evolutionary Microbiology. 52 (2): 297–354. doi:10.1099/00207713-52-2-297. PMID 11931142.
- Cavalier-Smith, Thomas (2009). "Megaphylogeny, Cell Body Plans, Adaptive Zones: Causes and Timing of Eukaryote Basal Radiations". Journal of Eukaryotic Microbiology. 56 (1): 26–33. doi:10.1111/j.1550-7408.2008.00373.x. PMID 19340985. S2CID 10205240.
- Harris, Elizabeth H. (2009) "The Genus Chlamydomonas" In The Chlamydomonas Sourcebook (Second Edition), chapter 1, volume 1, pages 1-24. ISBN 9780080919553 doi:10.1016/B978-0-12-370873-1.00001-0
- Jennings H. S. (1907) "Behavior of the Lower Organisms" The American Naturalist, 41(481): 42-44.
- Bahat, Anat; Tur-Kaspa, Ilan; Gakamsky, Anna; Giojalas, Laura C.; Breitbart, Haim; Eisenbach, Michael (2003). "Thermotaxis of mammalian sperm cells: A potential navigation mechanism in the female genital tract". Nature Medicine. 9 (2): 149–150. doi:10.1038/nm0203-149. PMID 12563318. S2CID 36538049.
- Sekiguchi, Masaya; Kameda, Shigetoshi; Kurosawa, Satoshi; Yoshida, Megumi; Yoshimura, Kenjiro (2018). "Thermotaxis in Chlamydomonas is brought about by membrane excitation and controlled by redox conditions". Scientific Reports. 8 (1): 16114. Bibcode:2018NatSR...816114S. doi:10.1038/s41598-018-34487-4. PMC 6208428. PMID 30382191.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Tawada, K.; Oosawa, F. (1972). "Responses of Parameciumto Temperature Change". The Journal of Protozoology. 19 (1): 53–57. doi:10.1111/j.1550-7408.1972.tb03412.x. PMID 5008849.
- Nakaoka, Yasuo; Oosawa, Fumio (1977). "Temperature-Sensitive Behavior of Paramecium caudatum". The Journal of Protozoology. 24 (4): 575–580. doi:10.1111/j.1550-7408.1977.tb01018.x.
- Hennessey, Todd M.; Saimi, Yoshiro; Kung, Ching (1983). "A heat-induced depolarization of Paramecium and its relationship to thermal avoidance behavior". Journal of Comparative Physiology A. 153: 39–46. doi:10.1007/BF00610340. S2CID 7152549.
- Pérez-Cerezales, Serafín; Boryshpolets, Sergii; Afanzar, Oshri; Brandis, Alexander; Nevo, Reinat; Kiss, Vladimir; Eisenbach, Michael (2015). "Involvement of opsins in mammalian sperm thermotaxis". Scientific Reports. 5: 16146. Bibcode:2015NatSR...516146P. doi:10.1038/srep16146. PMC 4633616. PMID 26537127.
- Hamano, Koh-Ichi; Kawanishi, Tae; Mizuno, Atsuko; Suzuki, Makoto; Takagi, Yuji (2016). "Involvement of Transient Receptor Potential Vanilloid (TRPV) 4 in mouse sperm thermotaxis". Journal of Reproduction and Development. 62 (4): 415–422. doi:10.1262/jrd.2015-106. PMC 5004798. PMID 27180924.
- De Blas, Gerardo A.; Darszon, Alberto; Ocampo, Ana Y.; Serrano, Carmen J.; Castellano, Laura E.; Hernández-González, Enrique O.; Chirinos, Mayel; Larrea, Fernando; Beltrán, Carmen; Treviño, Claudia L. (2009). "TRPM8, a Versatile Channel in Human Sperm". PLOS ONE. 4 (6): e6095. Bibcode:2009PLoSO...4.6095D. doi:10.1371/journal.pone.0006095. PMC 2705237. PMID 19582168.
- Kloppstech, Klaus; Meyer, Gabriele; Schuster, Gadi; Ohad, Itzhak (1985). "Synthesis, transport and localization of a nuclear coded 22-kd heat-shock protein in the chloroplast membranes of peas and Chlamydomonas reinhardi". The EMBO Journal. 4 (8): 1901–1909. doi:10.1002/j.1460-2075.1985.tb03869.x. PMC 554439. PMID 16453628.
- Van Lis, Robert; Atteia, Ariane; Mendoza-HernáNdez, Guillermo; GonzáLez-Halphen, Diego (2003). "Identification of Novel Mitochondrial Protein Components of Chlamydomonas reinhardtii. A Proteomic Approach". Plant Physiology. 132 (1): 318–330. doi:10.1104/pp.102.018325. PMC 166977. PMID 12746537.
- von Gromoff, E. D.; Treier, U.; Beck, C. F. (1989). "Three light-inducible heat shock genes of Chlamydomonas reinhardtii". Molecular and Cellular Biology. 9 (9): 3911–3918. doi:10.1128/mcb.9.9.3911-3918.1989. PMC 362453. PMID 2779571.
- Schroda, Michael; Hemme, Dorothea; Mühlhaus, Timo (2015). "The Chlamydomonasheat stress response". The Plant Journal. 82 (3): 466–480. doi:10.1111/tpj.12816. PMID 25754362.
- Valledor, Luis; Furuhashi, Takeshi; Hanak, Anne-Mette; Weckwerth, Wolfram (2013). "Systemic Cold Stress Adaptation of Chlamydomonas reinhardtii". Molecular & Cellular Proteomics. 12 (8): 2032–2047. doi:10.1074/mcp.M112.026765. PMC 3734567. PMID 23564937.
- Clegg, Mark R.; Maberly, Stephen C.; Jones, Roger I. (2003). "Behavioural responses of freshwater phytoplanktonic flagellates to a temperature gradient". European Journal of Phycology. 38 (3): 195–203. doi:10.1080/0967026031000121697. S2CID 85353895.
- Isogai, Nahoko; Kamiya, Ritsu; Yoshimura, Kenjiro (2000). "Dominance between the Two Flagella during Phototactic Turning in Chlamydomonas". Zoological Science. 17 (9): 1261–1266. doi:10.2108/zsj.17.1261. S2CID 84890095.
- Horst, C. J.; Witman, G. B. (1993). "Ptx1, a nonphototactic mutant of Chlamydomonas, lacks control of flagellar dominance". Journal of Cell Biology. 120 (3): 733–741. doi:10.1083/jcb.120.3.733. PMC 2119553. PMID 8425899.
- Okita, Noriko; Isogai, Nahoko; Hirono, Masafumi; Kamiya, Ritsu; Yoshimura, Kenjiro (2005). "Phototactic activity in Chlamydomonas non-phototactic' mutants deficient in Ca2+-dependent control of flagellar dominance or in inner-arm dynein". Journal of Cell Science. 118 (3): 529–537. doi:10.1242/jcs.01633. PMID 15657081. S2CID 2379702.
- Wakabayashi, K.-i.; Misawa, Y.; Mochiji, S.; Kamiya, R. (2011). "Reduction-oxidation poise regulates the sign of phototaxis in Chlamydomonas reinhardtii". Proceedings of the National Academy of Sciences. 108 (27): 11280–11284. Bibcode:2011PNAS..10811280W. doi:10.1073/pnas.1100592108. PMC 3131381. PMID 21690384.
- Wan, Kirsty Y.; Jékely, Gáspár (2021). "Origins of eukaryotic excitability". Philosophical Transactions of the Royal Society B: Biological Sciences. 376 (1820). arXiv:2007.13388. doi:10.1098/rstb.2019.0758. PMC 7935092. PMID 33487111.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Foster, K. W.; Smyth, R. D. (1980). "Light Antennas in phototactic algae". Microbiological Reviews. 44 (4): 572–630. doi:10.1128/MR.44.4.572-630.1980. PMC 373196. PMID 7010112.
- Holland, E.M.; Harz, H.; Uhl, R.; Hegemann, P. (1997). "Control of phobic behavioral responses by rhodopsin-induced photocurrents in Chlamydomonas". Biophysical Journal. 73 (3): 1395–1401. Bibcode:1997BpJ....73.1395H. doi:10.1016/S0006-3495(97)78171-2. PMC 1181038. PMID 9284306.
- Hayashi, Masahito; Yagi, Toshiki; Yoshimura, Kenjiro; Kamiya, Ritsu (1998). "Real-time observation of Ca2+-induced basal body reorientation in Chlamydomonas". Cell Motility and the Cytoskeleton. 41 (1): 49–56. doi:10.1002/(SICI)1097-0169(1998)41:1<49::AID-CM4>3.0.CO;2-A. PMID 9744298.
- Moriyama, Yasushige; Hiyama, Shigeo; Asai, Hiroshi (1998). "High-Speed Video Cinematographic Demonstration of Stalk and Zooid Contraction of Vorticella convallaria". Biophysical Journal. 74 (1): 487–491. Bibcode:1998BpJ....74..487M. doi:10.1016/s0006-3495(98)77806-3. PMC 1299401. PMID 9449349.
- Ando, Motonori; Shigenaka, Yoshinobu (1989). "Structure and function of the cytoskeleton in heliozoa: I. Mechanism of rapid axopodial contraction in Echinosphaerium". Cell Motility and the Cytoskeleton. 14 (2): 288–301. doi:10.1002/cm.970140214.
- Brunet, Thibaut; Arendt, Detlev (2016). "From damage response to action potentials: Early evolution of neural and contractile modules in stem eukaryotes". Philosophical Transactions of the Royal Society B: Biological Sciences. 371 (1685). doi:10.1098/rstb.2015.0043. PMC 4685582. PMID 26598726.
- Eckert, R.; Brehm, P. (1979). "Ionic Mechanisms of Excitation in Paramecium". Annual Review of Biophysics and Bioengineering. 8: 353–383. doi:10.1146/annurev.bb.08.060179.002033. PMID 383005.
- Eckert, Roger; Naitoh, Yutaka (1972). "Bioelectric Control of Locomotion in the Ciliates*†". The Journal of Protozoology. 19 (2): 237–243. doi:10.1111/j.1550-7408.1972.tb03444.x. PMID 4624297.
- Wood, David C. (1982). "Membrane permeabilities determining resting, action and mechanoreceptor potentials in Stentor coeruleus". Journal of Comparative Physiology A. 146 (4): 537–550. doi:10.1007/bf00609450. S2CID 21083419.
- Taylor, Alison R. (2009). "A Fast Na+/Ca2+-Based Action Potential in a Marine Diatom". PLOS ONE. 4 (3): e4966. Bibcode:2009PLoSO...4.4966T. doi:10.1371/journal.pone.0004966. PMC 2654917. PMID 19305505.
- Helliwell, Katherine E.; Chrachri, Abdul; Koester, Julie A.; Wharam, Susan; Verret, Frédéric; Taylor, Alison R.; Wheeler, Glen L.; Brownlee, Colin (2019). "Alternative Mechanisms for Fast Na+/Ca2+ Signaling in Eukaryotes via a Novel Class of Single-Domain Voltage-Gated Channels". Current Biology. 29 (9): 1503–1511.e6. doi:10.1016/j.cub.2019.03.041. PMC 6509283. PMID 31006567.
- Bingley, M.S.; Thompson, C.M. (1962). "Bioelectric potentials in relation to movement in amoebae". Journal of Theoretical Biology. 2 (1): 16–32. Bibcode:1962JThBi...2...16B. doi:10.1016/s0022-5193(62)80024-1.
- Harz, Hartmann; Hegemann, Peter (1991). "Rhodopsin-regulated calcium currents in Chlamydomonas". Nature. 351 (6326): 489–491. Bibcode:1991Natur.351..489H. doi:10.1038/351489a0. S2CID 4309593.
- Fujiu, Kenta; Nakayama, Yoshitaka; Iida, Hidetoshi; Sokabe, Masahiro; Yoshimura, Kenjiro (2011). "Mechanoreception in motile flagella of Chlamydomonas". Nature Cell Biology. 13 (5): 630–632. doi:10.1038/ncb2214. PMID 21478860. S2CID 19883187.
- Fujiu, Kenta; Nakayama, Yoshitaka; Yanagisawa, Ayaka; Sokabe, Masahiro; Yoshimura, Kenjiro (2009). "Chlamydomonas CAV2 Encodes a Voltage- Dependent Calcium Channel Required for the Flagellar Waveform Conversion". Current Biology. 19 (2): 133–139. doi:10.1016/j.cub.2008.11.068. PMID 19167228. S2CID 14063142.
- Umbach JA (1981) "pH and membrane excitability in Paramecium caudatum". Los Angeles, CA: University of California.
- Dunlap, K. (1977). "Localization of calcium channels in Paramecium caudatum". The Journal of Physiology. 271 (1): 119–133. doi:10.1113/jphysiol.1977.sp011993. PMC 1353610. PMID 915829.
- Echevarria, Michael L.; Wolfe, Gordon V.; Taylor, Alison R. (2015). "Feast or flee: Bioelectrical regulation of feeding and predator evasion behaviors in the planktonic alveolate Favella sp. (Spirotrichia)". Journal of Experimental Biology. 219 (Pt 3): 445–456. doi:10.1242/jeb.121871. PMID 26567352. S2CID 37255456.
- Lueken, Wolfgang; Ricci, Nicola; Krüppel, Thomas (1996). "Rhythmic spontaneous depolarizations determine a slow-and-fast rhythm in walking of the marine hypotrich Euplotes vannus". European Journal of Protistology. 32: 47–54. doi:10.1016/s0932-4739(96)80038-1.
- Kunita, Itsuki; Kuroda, Shigeru; Ohki, Kaito; Nakagaki, Toshiyuki (2014). "Attempts to retreat from a dead-ended long capillary by backward swimming in Paramecium". Frontiers in Microbiology. 5: 270. doi:10.3389/fmicb.2014.00270. PMC 4052044. PMID 24966852.
- Stock C, KrÜPpel T, Key G, Lueken W (1999) "Sexual behaviour in Euplotes raikovi is accompanied by pheromone-induced modifications of ionic currents". J Exp Biol, 202 (4): 475–483. PMID 9914154.
- Kimball RF (1942) "The Nature and Inheritance of Mating Types in Euplotes Patella". Genetics, 27(3): 269–285. PMID 17247040, PMC PMC1209158.
- Wood, DC (1988). "Habituation in Stentor: Produced by mechanoreceptor channel modification". The Journal of Neuroscience. 8 (7): 2254–2258. doi:10.1523/JNEUROSCI.08-07-02254.1988. PMC 6569508. PMID 3249223.
- Jennings, H. S. (1899). "Studies on Reactions to Stimuli in Unicellular Organisms. III Reactions to Localized Stimuli in Spirostomum and Stentor". The American Naturalist. 33 (389): 373–389. doi:10.1086/277256. S2CID 85272784.
- Dexter, Joseph P.; Prabakaran, Sudhakaran; Gunawardena, Jeremy (2019). "A Complex Hierarchy of Avoidance Behaviors in a Single-Cell Eukaryote". Current Biology. 29 (24): 4323–4329.e2. doi:10.1016/j.cub.2019.10.059. PMID 31813604. S2CID 208652463.
- Akolpoglu, Mukrime Birgul; Dogan, Nihal Olcay; Bozuyuk, Ugur; Ceylan, Hakan; Kizilel, Seda; Sitti, Metin (2020). "High‐Yield Production of Biohybrid Microalgae for On‐Demand Cargo Delivery". Advanced Science. 7 (16). doi:10.1002/advs.202001256. PMC 7435244. PMID 32832367.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Schwarz, Lukas; Medina-Sánchez, Mariana; Schmidt, Oliver G. (2017). "Hybrid Bio Micromotors". Applied Physics Reviews. 4 (3): 031301. Bibcode:2017ApPRv...4c1301S. doi:10.1063/1.4993441.
- Bastos-Arrieta, Julio; Revilla-Guarinos, Ainhoa; Uspal, William E.; Simmchen, Juliane (2018). "Bacterial Biohybrid Microswimmers". Frontiers in Robotics and AI. 5: 97. doi:10.3389/frobt.2018.00097. PMC 7805739. PMID 33500976.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Montemagno, Carlo; Bachand, George (1999). "Constructing nanomechanical devices powered by biomolecular motors". Nanotechnology. 10 (3): 225–231. Bibcode:1999Nanot..10..225M. doi:10.1088/0957-4484/10/3/301.
- Ricotti, Leonardo; Trimmer, Barry; Feinberg, Adam W.; Raman, Ritu; Parker, Kevin K.; Bashir, Rashid; Sitti, Metin; Martel, Sylvain; Dario, Paolo; Menciassi, Arianna (2017). "Biohybrid actuators for robotics: A review of devices actuated by living cells". Science Robotics. 2 (12): eaaq0495. doi:10.1126/scirobotics.aaq0495. PMID 33157905. S2CID 29776467.
- Alapan, Yunus; Yasa, Oncay; Yigit, Berk; Yasa, I. Ceren; Erkoc, Pelin; Sitti, Metin (2019). "Microrobotics and Microorganisms: Biohybrid Autonomous Cellular Robots". Annual Review of Control, Robotics, and Autonomous Systems. 2: 205–230. doi:10.1146/annurev-control-053018-023803. S2CID 139819519.
- Chu, Dafeng; Dong, Xinyue; Shi, Xutong; Zhang, Canyang; Wang, Zhenjia (2018). "Neutrophil-Based Drug Delivery Systems". Advanced Materials. 30 (22): e1706245. doi:10.1002/adma.201706245. PMC 6161715. PMID 29577477.
- Carlsen, Rika Wright; Sitti, Metin (2014). "Bio-Hybrid Cell-Based Actuators for Microsystems". Small. 10 (19): 3831–3851. doi:10.1002/smll.201400384. PMID 24895215.
- Nguyen, Van Du; Han, Ji-Won; Choi, Young Jin; Cho, Sunghoon; Zheng, Shaohui; Ko, Seong Young; Park, Jong-Oh; Park, Sukho (2016). "Active tumor-therapeutic liposomal bacteriobot combining a drug (Paclitaxel)-encapsulated liposome with targeting bacteria (Salmonella Typhimurium)". Sensors and Actuators B: Chemical. 224: 217–224. doi:10.1016/j.snb.2015.09.034.
- Felfoul, Ouajdi; Mohammadi, Mahmood; Taherkhani, Samira; De Lanauze, Dominic; Zhong Xu, Yong; Loghin, Dumitru; Essa, Sherief; Jancik, Sylwia; Houle, Daniel; Lafleur, Michel; Gaboury, Louis; Tabrizian, Maryam; Kaou, Neila; Atkin, Michael; Vuong, Té; Batist, Gerald; Beauchemin, Nicole; Radzioch, Danuta; Martel, Sylvain (2016). "Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions". Nature Nanotechnology. 11 (11): 941–947. Bibcode:2016NatNa..11..941F. doi:10.1038/nnano.2016.137. PMC 6094936. PMID 27525475.
- Yasa, Oncay; Erkoc, Pelin; Alapan, Yunus; Sitti, Metin (2018). "Microalga-Powered Microswimmers toward Active Cargo Delivery". Advanced Materials. 30 (45): e1804130. doi:10.1002/adma.201804130. PMID 30252963. S2CID 52823884.
- Ceylan, Hakan; Giltinan, Joshua; Kozielski, Kristen; Sitti, Metin (2017). "Mobile microrobots for bioengineering applications". Lab on a Chip. 17 (10): 1705–1724. doi:10.1039/C7LC00064B. PMID 28480466.
- Li, Jinxing; Esteban-Fernández De Ávila, Berta; Gao, Wei; Zhang, Liangfang; Wang, Joseph (2017). "Micro/Nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification". Science Robotics. 2 (4): eaam6431. doi:10.1126/scirobotics.aam6431. PMC 6759331. PMID 31552379.
- Erkoc, Pelin; Yasa, Immihan C.; Ceylan, Hakan; Yasa, Oncay; Alapan, Yunus; Sitti, Metin (2019). "Mobile Microrobots for Active Therapeutic Delivery". Advanced Therapeutics. 2. doi:10.1002/adtp.201800064. S2CID 88204894.
- Park, Byung-Wook; Zhuang, Jiang; Yasa, Oncay; Sitti, Metin (2017). "Multifunctional Bacteria-Driven Microswimmers for Targeted Active Drug Delivery". ACS Nano. 11 (9): 8910–8923. doi:10.1021/acsnano.7b03207. PMID 28873304.
- Behkam, Bahareh; Sitti, Metin (2007). "Bacterial flagella-based propulsion and on/Off motion control of microscale objects". Applied Physics Letters. 90 (2): 023902. Bibcode:2007ApPhL..90b3902B. doi:10.1063/1.2431454.
- Behkam, Bahareh; Sitti, Metin (2008). "Effect of quantity and configuration of attached bacteria on bacterial propulsion of microbeads". Applied Physics Letters. 93 (22): 223901. Bibcode:2008ApPhL..93v3901B. doi:10.1063/1.3040318.
- Mostaghaci, Babak; Yasa, Oncay; Zhuang, Jiang; Sitti, Metin (2017). "Bioadhesive Bacterial Microswimmers for Targeted Drug Delivery in the Urinary and Gastrointestinal Tracts". Advanced Science. 4 (6). doi:10.1002/advs.201700058. PMC 5473323. PMID 28638787.
- Schauer, Oliver; Mostaghaci, Babak; Colin, Remy; Hürtgen, Daniel; Kraus, David; Sitti, Metin; Sourjik, Victor (2018). "Motility and chemotaxis of bacteria-driven microswimmers fabricated using antigen 43-mediated biotin display". Scientific Reports. 8 (1): 9801. Bibcode:2018NatSR...8.9801S. doi:10.1038/s41598-018-28102-9. PMC 6023875. PMID 29955099.
- Singh, Ajay Vikram; Hosseinidoust, Zeinab; Park, Byung-Wook; Yasa, Oncay; Sitti, Metin (2017). "Microemulsion-Based Soft Bacteria-Driven Microswimmers for Active Cargo Delivery". ACS Nano. 11 (10): 9759–9769. doi:10.1021/acsnano.7b02082. PMID 28858477.
- Stanton, Morgan M.; Park, Byung-Wook; Miguel-López, Albert; Ma, Xing; Sitti, Metin; Sánchez, Samuel (2017). "Biohybrid Microtube Swimmers Driven by Single Captured Bacteria". Small. 13 (19). doi:10.1002/smll.201603679. hdl:2445/123481. PMID 28299891.
- Stanton, Morgan M.; Park, Byung-Wook; Vilela, Diana; Bente, Klaas; Faivre, Damien; Sitti, Metin; Sánchez, Samuel (2017). "Magnetotactic Bacteria Powered Biohybrids TargetE. Coli Biofilms". ACS Nano. 11 (10): 9968–9978. doi:10.1021/acsnano.7b04128. hdl:2445/123493. PMID 28933815.
- Harris, Elizabeth H. (2001). "Chlamydomonasas Amodelorganism". Annual Review of Plant Physiology and Plant Molecular Biology. 52: 363–406. doi:10.1146/annurev.arplant.52.1.363. PMID 11337403.
- Weibel, D. B.; Garstecki, P.; Ryan, D.; Diluzio, W. R.; Mayer, M.; Seto, J. E.; Whitesides, G. M. (2005). "Microoxen: Microorganisms to move microscale loads". Proceedings of the National Academy of Sciences. 102 (34): 11963–11967. Bibcode:2005PNAS..10211963W. doi:10.1073/pnas.0505481102. PMC 1189341. PMID 16103369.
- Hopfner, Ursula; Schenck, Thilo-Ludwig; Chávez, Myra-Noemi; Machens, Hans-Günther; Bohne, Alexandra-Viola; Nickelsen, Jörg; Giunta, Riccardo-Enzo; Egaña, José-Tomás (2014). "Development of photosynthetic biomaterials for in vitro tissue engineering". Acta Biomaterialia. 10 (6): 2712–2717. doi:10.1016/j.actbio.2013.12.055. PMID 24406198.
- Centeno-Cerdas, Carolina; Jarquín-Cordero, Montserrat; Chávez, Myra Noemi; Hopfner, Ursula; Holmes, Christopher; Schmauss, Daniel; Machens, Hans-Günther; Nickelsen, Jörg; Egaña, José Tomás (2018). "Development of photosynthetic sutures for the local delivery of oxygen and recombinant growth factors in wounds". Acta Biomaterialia. 81: 184–194. doi:10.1016/j.actbio.2018.09.060. PMID 30287280. S2CID 52922420.
- Schenck, Thilo Ludwig; Hopfner, Ursula; Chávez, Myra Noemi; Machens, Hans-Günther; Somlai-Schweiger, Ian; Giunta, Riccardo Enzo; Bohne, Alexandra Viola; Nickelsen, Jörg; Allende, Miguel L.; Egaña, José Tomás (2015). "Photosynthetic biomaterials: A pathway towards autotrophic tissue engineering". Acta Biomaterialia. 15: 39–47. doi:10.1016/j.actbio.2014.12.012. PMID 25536030.
- Ng, Wei Ming; Che, Hui Xin; Guo, Chen; Liu, Chunzhao; Low, Siew Chun; Chieh Chan, Derek Juinn; Mohamud, Rohimah; Lim, Jitkang (2018). "Artificial Magnetotaxis of Microbot: Magnetophoresis versus Self-Swimming". Langmuir. 34 (27): 7971–7980. doi:10.1021/acs.langmuir.8b01210. PMID 29882671. S2CID 46953567.
Further reading
- Cohn, Stanley; Manoylov, Kalina; Gordon, Richard (2021). Diatom gliding motility : biology and applications. Beverly, MA: Scrivener Publishing. ISBN 978-1-119-52648-3. OCLC 1262966612.
