Tezepelumab
Tezepelumab, sold under the brand name Tezspire, is a human monoclonal antibody used for the treatment of asthma.[3][5][6][7] Tezepelumab blocks thymic stromal lymphopoietin (TSLP),[3] an epithelial cytokine that has been suggested to be critical in the initiation and persistence of airway inflammation.[8]
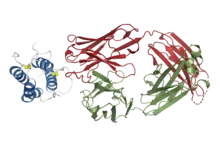 Structural basis for inhibition of TSLP-signaling by Tezepelumab (PDB 5J13)[1] | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | thymic stromal lymphopoietin (TSLP) |
| Clinical data | |
| Trade names | Tezspire |
| Other names | MEDI9929, AMG 157, tezepelumab-ekko |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6400H9844N1732O1992S52 |
| Molar mass | 144590.40 g·mol−1 |
The most common side effects include arthralgia (joint pain) and pharyngitis (sore throat).[5]
Tezepelumab was approved for medical use in the United States in December 2021,[3][4] and in the European Union in September 2022.[5][9]
Medical uses
Tezepelumab is indicated for the add-on maintenance treatment of people aged twelve years and older with severe asthma.[3][5]
History
Two main studies including over 1,500 adults and adolescents with inadequately controlled asthma showed that tezepelumab was effective in reducing the number of severe asthma flare‑ups.[5]
Society and culture
Legal status
On 21 July 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Tezspire, intended as add-on treatment in adult and adolescent patients with severe asthma.[5][10] The applicant for this medicinal product is AstraZeneca AB.[10] Tezepelumab was approved for medical use in the European Union in September 2022.[5]
Research
It is being studied for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, chronic spontaneous urticaria and eosinophilic esophagitis (EoE).[4]
In phase III trials, tezepelumab demonstrated efficacy compared to placebo for patients with severe, uncontrolled asthma.[11][12]
Structural studies by X-ray crystallography showed that tezepelumab competes against a critical part of the TSLPR binding site on TSLP.[1]
References
- Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. (April 2017). "Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma". Nature Communications. 8 (1): 14937. Bibcode:2017NatCo...814937V. doi:10.1038/ncomms14937. PMC 5382266. PMID 28368013.
- "Tezspire Product information". Health Canada. 25 April 2012. Retrieved 1 October 2022.
- "Tezspire- tezepelumab-ekko injection, solution". DailyMed. Archived from the original on 25 December 2021. Retrieved 24 December 2021.
- "Tezspire (tezepelumab) approved in the US for severe asthma". AstraZeneca (Press release). 17 December 2021. Archived from the original on 18 December 2021. Retrieved 17 December 2021.
- "Tezspire EPAR". European Medicines Agency. 19 July 2022. Archived from the original on 22 September 2022. Retrieved 21 September 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Marone G, Spadaro G, Braile M, Poto R, Criscuolo G, Pahima H, et al. (November 2019). "Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma". Expert Opinion on Investigational Drugs. 28 (11): 931–940. doi:10.1080/13543784.2019.1672657. PMID 31549891. S2CID 202746054.
- Matera MG, Rogliani P, Calzetta L, Cazzola M (February 2020). "TSLP Inhibitors for Asthma: Current Status and Future Prospects". Drugs. 80 (5): 449–458. doi:10.1007/s40265-020-01273-4. PMID 32078149. S2CID 211194472.
- "Tezepelumab granted Breakthrough Therapy Designation by US FDA". AstraZeneca (Press release). 7 September 2018. Archived from the original on 10 September 2018. Retrieved 10 September 2018.
- "Tezspire approved in the EU for the treatment of severe asthma" (Press release). AstraZeneca. 21 September 2022. Archived from the original on 22 September 2022. Retrieved 21 September 2022.
- "Tezspire: Pending EC decision". European Medicines Agency. 21 July 2022. Archived from the original on 28 July 2022. Retrieved 30 July 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Studies found for: Tezepelumab". ClinicalTrials.Gov. National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services. Archived from the original on 30 July 2022. Retrieved 14 March 2020.
- Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. (May 2021). "Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma". New England Journal of Medicine. 384 (19): 1800–09. doi:10.1056/NEJMoa2034975. PMID 33979488. S2CID 234484931. Archived from the original on 28 May 2022. Retrieved 29 March 2022.
External links
- "Tezepelumab". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02054130 for "Study to Evaluate the Efficacy and Safety of MEDI9929 (AMG 157) in Adult Subjects With Inadequately Controlled, Severe Asthma" at ClinicalTrials.gov
- Clinical trial number NCT03347279 for "Study to Evaluate Tezepelumab in Adults & Adolescents With Severe Uncontrolled Asthma (NAVIGATOR)" at ClinicalTrials.gov