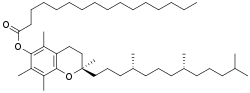
Α-Tocopheryl palmitate
 | |
| Clinical data | |
|---|---|
| Other names | α-Tocopherol palmitate |
| Routes of administration | Intramuscular injection |
| Drug class | Vitamin E |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.340 |
| Chemical and physical data | |
| Formula | C45H80O3 |
| Molar mass | 669.132 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
α-Tocopheryl palmitate is the palmitate ester of α-tocopherol and is a form of vitamin E.[1][2] Related compounds include α-tocopheryl acetate and α-tocopheryl succinate.
See also
References
- ↑ Baxter JG, Robeson CD, Taylor JD, Lehman RW (1943). "Natural α-, β- and γ-Tocopherols and Certain Esters of Physiological Interest1". Journal of the American Chemical Society. 65 (5): 918–924. doi:10.1021/ja01245a041. ISSN 0002-7863.
- ↑ Ben-Shabat S, Kazdan Y, Beit-Yannai E, Sintov AC (May 2013). "Use of alpha-tocopherol esters for topical vitamin E treatment: evaluation of their skin permeation and metabolism". The Journal of Pharmacy and Pharmacology. 65 (5): 652–8. doi:10.1111/jphp.12027. PMID 23600381. S2CID 31085958.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.