Fungal isolate
Fungal isolates have been researched for decades. Because fungi often exist in thin mycelial monolayers, with no protective shell, immune system, and limited mobility, they have developed the ability to synthesize a variety of unusual compounds for survival. Researchers have discovered fungal isolates with anticancer, antimicrobial, immunomodulatory, and other bio-active properties. The first statins, β-Lactam antibiotics, as well as a few important antifungals, were discovered in fungi.
Chemotherapeutic isolates
BMS manufactures paclitaxel using Penicillium and plant cell fermentation. Fungi can synthesize podophyllotoxin and camptothecin, precursors to etoposide, teniposide, topotecan, and irinotecan.
Lentinan, PSK, and PSP, are registered anticancer immunologic adjuvants. Irofulven and acylfulvene are anticancer derivatives of illudin S. Clavaric acid is a reversible farnesyltransferase inhibitor. Inonotus obliquus creates betulinic acid precursor betulin. Flammulina velutipes creates asparaginase. Plinabulin is a fungal isolate derivative currently being researched for anticancer applications.
Cholesterol inhibitors
The statins lovastatin, mevastatin, and simvastatin precursor monacolin J, are fungal isolates. Additional fungal isolates that inhibit cholesterol are zaragozic acids, eritadenine, and nicotinamide riboside.
Immunosuppressants
Ciclosporin, mycophenolic acid, mizoribine, and gliotoxin, are immunosuppressant fungal isolates.
Antimicrobials
Penicillin, cephalosporins, fusafungine, usnic acid, fusidic acid, fumagillin, brefeldin A, verrucarin A, alamethicin, are antibiotic fungal isolates. Antibiotics retapamulin, tiamulin, and valnemulin are derivatives of the fungal isolate pleuromutilin. Griseofulvin, echinocandins, strobilurin, azoxystrobin, caspofungin, micafungin, are fungal isolates with antifungal activity.
Psychotropic isolates
The headache medications cafergot, dihydroergotamine, methysergide, methylergometrine, the dementia medications hydergine, nicergoline, the Parkinson's disease medications lisuride, bromocriptine, cabergoline, and pergolide were all derived from Claviceps isolates. Polyozellus multiplex synthesizes prolyl endopeptidase inhibitors polyozellin, thelephoric acid, and kynapcins. Boletus badius synthesizes L-theanine.
Other isolates
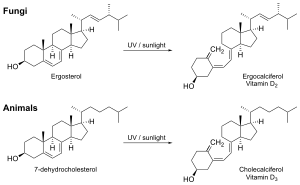
Researchers have discovered other interesting fungal isolates like the antihyperglycemic compounds ternatin, aspergillusol A, sclerotiorin, and antimalarial compounds codinaeopsin, efrapeptins, and antiamoebin. The fungal isolate ergothioneine is actively absorbed and concentrated by the human body via SLC22A4. Other notable fungal isolates include vitamin D1, vitamin D2, and vitamin D4.
| Isolate | Source | Researched activity / Chemical description |
|---|---|---|
| 9-Deacetoxyfumigaclavine C | endophytic Aspergillus fumigatus | potent, selective, anticancer activity comparable to doxorubicin (IC50 = 3.1 μM against K562)[1] |
| 14-Norpseurotin A | Aspergillus | antiparasitic/anticancer[1] |
| 3-O-Methylfunicone | Penicillium pinophilum | in vitro cancer stem cell inhibitor |
| Anicequol | Penicillium aurantiogriseum | in vitro anchorage-independent cancer inhibitor |
| Anomalin A | sponge-derived Arthrinium | angiogenesis inhibitor |
| Antiamoebin | Emericellopsis | anti-microbial/protozoan polypeptide |
| Arugosin C | Aspergillus versicolor isolated from Red Sea green alga | bio-active anthraquinone[2] |
| Aspergillides A-C | marine Aspergillus ostianus | anticancer/cytotoxic |
| Aspergillusene | "sea fan"-derived Aspergillus sydowii | antioxidant sesquiterpene |
| Aspergilone A | "sea fan"-derived Aspergillus | anticancer and antifouling activity[3] |
| Aspergillusol A | marine Aspergillus | alpha-glucosidase inhibitor |
| Asperterrestide A | marine Aspergillus terreus | cytotoxic and antiviral cyclic tetrapeptide |
| Asterric acid | Antarctic Geomyces | endothelin binding inhibitor |
| Auranthine | Penicillium | antimicrobial |
| Aurantiamine | Penicillium aurantiogriseum | valine and histidine derived diketopiperazine |
| Aurantiomide | sponge-derived Penicillium aurantiogriseum | quinazoline alkaloid with cytotoxic/anticancer activity |
| Berkeleydione | fungal extremophile (Berkeley Pit, Montana) | anticancer polyketide-terpenoid |
| Berkeleytrione | fungal extremophile (Berkeley Pit, Montana) | anticancer polyketide-terpenoid |
| Berkelic acid | fungal extremophile (Berkeley Pit, Montana) | spiroketal anticancer compound |
| beta-Ergocryptine | ergot | dopaminergic ergot alkaloid |
| Bisvertinolone | Trichoderma | anticancer[4] |
| Botryodiplodin | Penicillium | antibiotic mycotoxin |
| Brevianamide S | marine Aspergillus versicolor | antimicrobial dimeric diketopiperazine |
| Brevicompanines D-H | deep ocean sediment Penicillium | lipopolysaccharide (LPS)-induced nitric oxide inhibitor |
| Cephalosporolide | marine Penicillium | novel lactones |
| Chaetoglobosin A | Chaetomium | anticancer[5] |
| Chaetoxanthone | marine-derived Chaetomium | bio-active xanthone |
| Chanoclavine | ergot | dopamine agonist |
| Chanoclavine II | ergot | |
| Chetracins B | Antarctic psychrophilic Oidiodendron truncatum | in vitro anticancer (nanomolar) |
| Chrysophanic acid | antiviral/anticancer anthraquinone | |
| Citreorosein | Penicillium | antimicrobial polyketide |
| Citrinolactone D | marine-derived Penicillium | citrinin derivative |
| Citromycetin | Australian Penicillium | bio-active polyketide |
| Citromycin | Penicillium | antibiotic |
| Communesin B | Mediterranean Axinella-derived Penicillium | anticancer |
| Costaclavin | ergot | |
| Cryptoechinuline D | mangrove rhizosphere soil-derived Aspergillus | anticancer |
| Curvularin | Penicillium | antimicrobial |
| Decumbenone C | marine Aspergillus sulphureus | anticancer |
| Dehydroaltenusin | Alternaria tenuis | inhibitor of mammalian DNA polymerase α |
| Dehydrocurvularin | Penicillium | antimicrobial |
| Disydonols A-C | marine Aspergillus | anticancer |
| Duclauxin | Penicillium duclauxi | anticancer[6] |
| Epicoccins | Cordyceps-colonizing Epicoccum nigrum | antiviral |
| Epolactaene | marine fungus | antiinflammatory, inhibitory activity of DNA polymerases and DNA topoisomerase II, active synthetic analogs[7] |
| Epoxyagroclavine | permafrost Penicillium | ergot alkaloid |
| Epoxyphomalins A-B | marine Paraconiothyrium | potent cytotoxics |
| Ergosine | ergot | dopaminergic ergot alkaloid |
| Ergostane | mushrooms | steroid |
| Ergostine | ergot | alpha-adrenergic blocking, vasoconstrictive ergot alkaloid |
| Eupenifeldin | Eupenicillium brefeldianum | antimicrobial cytotoxic bistropolone |
| Evariquinone | Emericella variecolor (derived from the marine sponge Haliclona) | |
| Fecosterol | fungi and lichens | steroid |
| Fellutanine | Penicillium | bio-active diketopiperazine alkaloids |
| Festuclavine | Aspergillus fumigatus | bio-active ergoline |
| Fumigaclavine A | endophytic Aspergillus | bio-active ergoline |
| Fumigaclavine B | endophytic Aspergillus | bio-active ergoline |
| Fumigaclavine C | endophytic Aspergillus | bio-active ergoline |
| Fumiquinazoline | soft coral Sinularia-derived Aspergillus fumigatus | cytotoxic/anticancer |
| Fungisterol | Cordyceps sinensis | steroid |
| Glionitrin A | mine-dwelling Aspergillus fumigatus | antibiotic-anticancer |
| Glionitrin B | Aspergillus fumigatus KMC-901 | anticancer diketopiperazine |
| Hymenosetin | Hymenoscyphus pseudoalbidus | antimicrobial (active against MRSA)[8] |
| Isoemericellin | marine Emericella variecolor | |
| Leporizines A-C | Aspergillus | cytotoxic epithiodiketopiperazines |
| Leptosphaerin | marine Leptosphaeria oraemaris | antifungal |
| Lichesterol | fungi and lichens | steroid |
| Luteoalbusins A-B | deep sea Acrostalagmus luteoalbus | anticancer indole diketopiperazines |
| Luteusin A | Talaromyces luteus | monoamine oxidase inhibitor |
| Malettinin | Hypoxylon | polyketide/antimicrobial |
| Maximiscin | Tolypocladium (Salcha, Alaska)[9] | anticancer polyketide-shikimate compound |
| Meleagrin | deep ocean Penicillium | anticancer |
| Methylenolactocin | Penicillium | anticancer |
| Neoxaline | Aspergillus japonicus | antimitotic and antiplatelet |
| Nigerapyrones A-E | marine mangrove-derived, endophytic Aspergillus niger | anticancer |
| Nigrosporin B | Nigrospora | antimicrobial |
| Nocapyrones E-G | Nocardiopsis dassonvillei | antimicrobial alpha-pyrones |
| Notoamide | marine Aspergillus | bio-active prenylated indole alkaloid |
| Oxaline | Penicillium oxalicum and Aspergillus japonicus | anticancer (tubulin polymerization inhibitor), O-methylated derivative of meleagrin |
| Pencolide | seaweed-derived endophytic fungi | bio-active maleimide |
| Penicitrinol J | marine-derived Penicillium | bio-active citrinin dimer |
| Penicitrinol K | marine-derived Penicillium | bio-active citrinin derivative |
| Penicitrinone E | marine-derived Penicillium | bio-active citrinin dimer |
| Penochalasin A | endophytic Chaetomium | cytotoxic/anticancer cytochalasan-based alkaloid |
| Penostatin A | Penicillium | cytotoxic metabolite |
| Pestalamides A-C | Pestalotiopsis theae | antiviral and antifungal |
| Petrosifungin | sponge-derived Penicillium brevicompactum | novel cyclodepsipeptide |
| Phillyrin | endophytic fungus (isolated from Forsythia) | antiobesity |
| Piscarinine | Penicillium piscarium westling | bio-active polycyclic diketopiperazine alkaloid |
| Prenylterphenyllins | marine Aspergillus candidus | anticancer |
| Protuboxepins A and B | Aspergillus SF-5044 | anticancer diketopiperazines |
| Pseurotin A | endophytic Aspergillus | antiparasitic and anticancer |
| Pyrenocine | marine Penicillium paxilli | antibiotic/antiinflammatory mycotoxin[10] |
| Questiomycin A | Penicillium expansum | antibiotic |
| Quinocitrinine | permafrost Penicillium | quinoline alkaloid |
| RES-1149-2 | Aspergillus | non-peptidic endothelin receptor antagonist |
| Retigeric acid B | Lobaria (lichen) | anticancer |
| Rubratoxin B | Penicillium rubrum | anticancer |
| Rugulovasine | Penicillium | |
| Sch 642305 | Penicillium verrucosum and Rhizoctonia solani | bacterial DNA primase inhibitor |
| Sclerotides A-B | Aspergillus sclerotiorum PT06-1 | bio-active cyclic hexapeptides |
| Secalonic acid | marine fungi | nootropic |
| Shamixanthone | Aspergillus | bio-active prenylated xanthone |
| Shearinine | marine Penicillium janthinellum | anticancer |
| Siderin | Aspergillus versicolor isolated from Red Sea green alga | bio-active anthraquinone |
| Sorbicillactone A | sponge-derived fungus | novel bio-active alkaloid |
| Spiculisporic acid | marine Aspergillus | bioactive γ-butenolide |
| Spiropreussione | Preussia | anticancer |
| Stephacidin | Aspergillus ochraceus WC76466 | anticancer/cytotoxic |
| Stromemycin | marine Emericella | C-glycosidic depside matrix metalloproteinase inhibitor |
| Terpestacin | endophytic fungus Drechslera ravenelii | anticancer |
| Terrestrols | marine Penicillium terrestre | cytotoxic/anticancer[11] |
| Terreulactone A | Aspergillus terreus | anti-acetylcholinesterase terpenoid |
| Topopyrone C | Phoma and Penicillium | anticancer human topoisomerase I inhibitor |
| Trachyspic acid | Talaromyces trachyspermus | heparanase inhibitor |
| Trichodimerol | Trichoderma | bio-active pentacycle |
| Ustusolates | marine Aspergillus ustus | anticancer |
| Variecolactone | Emericella purpurea mycelium | immunomodulatory sesterterpene |
| Variecolol | Emericella aurantio-brunnea | immunosuppressant/antiviral alkaloid |
| Varixanthone | marine Emericella variecolor | antimicrobial |
| Vermiculine | Penicillium vermiculatum | antibiotic |
| Vermistatin | fungal extremophile (Berkeley Pit, Montana) | anticancer[12] |
| Vermixocin | Penicillium vermiculatum | cytotoxic metabolite |
| Verrucosidin | Penicillium verrucosum | cytotoxic pyrone-type polyketide |
| Verrulactone A | Penicillium | antimicrobial alternariol |
| Versicolamide B | marine Aspergillus | a paraherquamide-stephacidin |
| Viscumamide | mangrove-derived endophytic fungi | cyclic peptide |
| Yaequinolone J1 | Penicillium sp. FKI-2140 | antibiotic |
See also
- Bacillus isolates
- Biotechnology in pharmaceutical manufacturing
- Mycorrhiza
- Aspergillus oryzae, Saccharomyces cerevisiae, Saccharomyces boulardii
- Sponge isolates
- Streptomyces isolates
References
- 1 2 Ge HM, Yu ZG, Zhang J, Wu JH, Tan RX (2009). "Bioactive alkaloids from endophytic Aspergillus fumigatus". J Nat Prod. 72 (4): 753–5. doi:10.1021/np800700e. PMID 19256529.
- ↑ Hawas UW, El-Beih AA, El-Halawany AM (2012). "Bioactive anthraquinones from endophytic fungus Aspergillus versicolor isolated from red sea algae". Arch Pharm Res. 35 (10): 1749–56. doi:10.1007/s12272-012-1006-x. PMID 23139125.
- ↑ Shao CL, Wang CY, Wei MY, Gu YC, She ZG, Qian PY, et al. (2011). "Aspergilones A and B, two benzylazaphilones with an unprecedented carbon skeleton from the gorgonian-derived fungus Aspergillus sp". Bioorg Med Chem Lett. 21 (2): 690–3. doi:10.1016/j.bmcl.2010.12.005. PMID 21194945.
- ↑ Abe, N; Arakawa, T; Hirota, A (2002). "The biosynthesis of bisvertinolone: Evidence for oxosorbicillinol as a direct precursor". Chemical Communications (Cambridge, England) (3): 204–5. doi:10.1039/b109505f. PMID 12120368.
- ↑ Kawahara, T; Itoh, M; Izumikawa, M; Sakata, N; Tsuchida, T; Shin-Ya, K (2013). "New chaetoglobosin derivatives, MBJ-0038, MBJ-0039 and MBJ-0040, isolated from the fungus Chaetomium sp. f24230". The Journal of Antibiotics. 66 (12): 727–30. doi:10.1038/ja.2013.75. PMID 23881215.
- ↑ Kuhr, I; Fuska, J; Sedmera, P; Podojil, M; Vokoun, J; Vanĕk, Z (1973). "An antitumor antibiotic produced by Penicillium stipitatum Thom; its identity with duclauxin". The Journal of Antibiotics. 26 (9): 535–6. doi:10.7164/antibiotics.26.535. PMID 4799788.
- ↑ Mizushina Y, Kuramochi K, Ikawa H, Kuriyama I, Shimazaki N, Takemura M, et al. (2005). "Structural analysis of epolactaene derivatives as DNA polymerase inhibitors and anti-inflammatory compounds". Int J Mol Med. 15 (5): 785–93. doi:10.3892/ijmm.15.5.785. PMID 15806299.
- ↑ Halecker S, Surup F, Kuhnert E, Mohr KI, Brock NL, Dickschat JS, et al. (2014). "Hymenosetin, a 3-decalinoyltetramic acid antibiotic from cultures of the ash dieback pathogen, Hymenoscyphus pseudoalbidus". Phytochemistry. 100: 86–91. doi:10.1016/j.phytochem.2014.01.018. hdl:10033/337879. PMID 24529574.
- ↑ Du, L; Robles, AJ; King, JB; Powell, DR; Miller, AN; Mooberry, SL; Cichewicz, RH (2013). "Crowdsourcing Natural Products Discovery to Access Uncharted Dimensions of Fungal Metabolite Diversity". Angewandte Chemie International Edition in English. 53 (3): 804–9. doi:10.1002/anie.201306549. PMC 4028707. PMID 24285637.
- ↑ Toledo TR, Dejani NN, Monnazzi LG, Kossuga MH, Berlinck RG, Sette LD, et al. (2014). "Potent Anti-Inflammatory Activity of Pyrenocine A Isolated from the Marine-Derived Fungus Penicillium paxilli Ma(G)K". Mediators Inflamm. 2014: 767061. doi:10.1155/2014/767061. PMC 3916108. PMID 24574582.
- ↑ Chen L, Fang Y, Zhu T, Gu Q, Zhu W (2008). "Gentisyl alcohol derivatives from the marine-derived fungus Penicillium terrestre". J Nat Prod. 71 (1): 66–70. doi:10.1021/np070421v. PMID 18163588.
- ↑ Stierle AA, Stierle DB, Girtsman T (2012). "Caspase-1 inhibitors from an extremophilic fungus that target specific leukemia cell lines". J Nat Prod. 75 (3): 344–50. doi:10.1021/np200414c. PMC 3330824. PMID 22295871.