Theanine
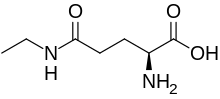 L-Theanine | |
| Clinical data | |
|---|---|
| Other names | γ-L-Glutamylethylamide |
| Dependence liability | None |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Onset of action | about 1 hour[1] |
| Elimination half-life | Capsule ~1.2 hours Green Tea ~0.8 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.019.436 |
| Chemical and physical data | |
| Formula | C7H14N2O3 |
| Molar mass | 174.200 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 174.20 °C (345.56 °F) [3] |
| Boiling point | 215 °C (419 °F) [3] |
SMILES
| |
InChI
| |
Theanine /ˈθiːəniːn/, also known as L-γ-glutamylethylamide and N5-ethyl-L-glutamine, is an amino acid analogue of the proteinogenic amino acids L-glutamate and L-glutamine and is found primarily in particular plant and fungal species. It was discovered as a constituent of green tea in 1949; in 1950, it was isolated from gyokuro leaves.[4] Theanine provides a unique brothy or savory (umami) flavor to green tea infusions.
The name "theanine" without a prefix generally implies the enantiomer L-theanine, which is the form found in tea leaves and as a dietary supplement ingredient. Most studies have used L-theanine. The opposite enantiomer, D-theanine, has been studied less.
The regulatory status of theanine varies by country. In Japan, L-theanine has been approved for use in all foods, with some restrictions in the case of infant foods.[5][6] In the United States, the Food and Drug Administration (FDA) considers it to be generally recognized as safe (GRAS) and allows its sale as a dietary supplement. The German Federal Institute for Risk Assessment, an agency of their Federal Ministry of Food and Agriculture, objects to the addition of L-theanine to beverages. The European Food Safety Authority, when asked to provide a scientific opinion, concluded that a cause and effect relationship had not been established between consumption of L-theanine and improved cognitive function, alleviation of psychological stress, maintenance of normal sleep, or reduction of menstrual discomfort.[7] Therefore, health claims for L-theanine are not recognized in the European Union.[8]
Structure and properties
The chemical name N5-ethyl-L-glutamine[2] and other synonyms (see box) for theanine reflect its chemical structure. The name theanine, without prefix, is generally understood to imply the L- (S-) enantiomer, derived from the related proteinogenic L-amino acid glutamic acid. Theanine is an analog of this amino acid, and its primary amide, L-glutamine (also a proteinogenic amino acid). Theanine is a derivative of glutamine that is ethylated on the amide nitrogen (as the name N5-ethyl-L-glutamine describes), or alternatively, to the amide formed from ethylamine and L-glutamic acid at its γ- (5-) side chain carboxylic acid group (as the name γ-L-glutamylethylamide describes).
Relative to theanine, the opposite (D-, R-) enantiomer is largely absent from the literature,[2] except implicitly. While natural extracts that are not harshly treated are presumed to contain only the biosynthetic L- enantiomeric form, mishandled isolates and racemic chemical preparations of theanines necessarily contain both theanine and its D-enantiomer (and from racemic syntheses, in equal proportion), and studies have suggested that the D-isomer may actually predominate in some commercial supplement preparations.[9][10] Amino acid racemization in aqueous media is a well-established chemical process promoted by elevated temperature and non-neutral pH values; prolonged heating of Camellia extracts—possible for oversteeped teas and in undisclosed commercial preparative processes—has been reported to result in increasing racemization of theanine to give increasing proportions of the nonnatural D-theanine, up to equal proportions of each enantiomer.[10]
Discovery and distribution
Theanine is found primarily in plant and fungal species. It was discovered as a constituent of tea (Camellia sinensis) in 1949, and in 1950 a laboratory in Kyoto[5] successfully isolated it from gyokuro leaf, which has high theanine content.[11] Theanine is substantially present in black, green, and white teas from Camellia sinensis in quantities of about 1% of the dry weight.[12][13] Deliberately shading tea plants from direct sunlight, as is done for matcha and gyokuro green tea, increases L-theanine content. The L-enantiomer[2] is the form found in freshly prepared teas and some human dietary supplements.[10]
Digestion and metabolism
As a structural analog of glutamate and glutamine, the theanine in preparations (teas, pure supplements, etc.) is absorbed in the small intestine after oral ingestion; its hydrolysis to L-glutamate and ethylamine occur both in the intestine and liver, so theanine can be considered to function as a donor that supplies glutamate to the body.[14] Glutamate can be metabolized to glutamine in astrocytes, a process catalysed by Glutamine synthetase and can also be decarboxylated to GABA by Glutamate decarboxylase, thus theanine can supply the neurotransmitter pools of amino acids.[15] It can also cross the blood–brain barrier intact, and register pharmacological effects directly.[16]
Pharmacology
Pharmacodynamics
Theanine is structurally similar to the excitatory neurotransmitter glutamate, and in accordance, binds to glutamate receptors, though with much lower affinity in comparison. Specifically, it binds to ionotropic glutamate receptors in the micromolar range, including the AMPA and kainate receptors and, to a lesser extent, the NMDA receptor.[17][18][19][20] It acts as an antagonist of the former two sites, and a partial co-agonist of the NMDA receptors.[20][21] Theanine also binds to group I mGluRs.[17][22] In addition, it inhibits glutamine transporters and glutamate transporters, and thus blocks the reuptake of glutamine and glutamate.[19][23][24] Lastly, theanine elicits umami taste, and this effect has been found to be a consequence of the fact that it directly binds to and activates the T1R1 + T1R3 heterodimer or umami (savory) taste receptor.[25]
Theanine increases serotonin, dopamine and glycine levels in various areas of the brain, as well as BDNF and NGF levels in certain brain areas.[17][26][27][28] However, its effect on serotonin is still a matter of debate in the scientific community, with studies showing increases and decreases in brain serotonin levels using similar experimental protocols.[16][29] It has also been found that injecting spontaneously hypertensive mice with theanine significantly lowered levels of 5-hydroxyindoles in the brain.[30] Researchers also speculate that it may inhibit glutamate excitotoxicity.[17]
Effects
A Natural Standard monograph that reviews current research on theanine reports that it is likely safe in doses of 200–250 mg up to a maximum daily dose of 1,200 mg. Natural Standard rates the evidence to support the usage of theanine for anxiety reduction, blood pressure control, and mood improvement as "unclear or conflicting scientific evidence" and the evidence for improved cognition as "fair negative scientific evidence". Many of the studies of theanine were done in combination with caffeine as found in tea. While the studies found that the combination had some effect on mood, the studies found that theanine alone had little effect.[31] A review by other researchers of a small set of trials concluded that there are benefits of L-theanine in reducing acute stress and anxiety in people with stressful conditions.[32]
Supplement use

A 2020 systematic review concluded that L-theanine supplementation between 200 and 400 mg per day may help reduce stress and anxiety acutely in people with acute stress, but there is insufficient evidence for treatment of chronic stress. It further concluded that longer term and larger clinical study is needed to justify its use therapeutically.[33]
In 2001, the German Federal Institute for Risk Assessment (Bundesinstitut für Risikobewertung, BfR) objected to the addition of isolated theanine to beverages.[34][35] The institute stated the amount of theanine consumed by regular drinkers of tea or coffee is virtually impossible to determine. While it was estimated the quantity of green tea consumed by the average Japanese tea drinker per day contains about 20 mg of the substance, there are no studies measuring the amount of theanine being extracted by typical preparation methods, or the percentage lost by discarding the first infusion. Therefore, with the Japanese being exposed to possibly much less than 20 mg per day, and Europeans presumably even less, it was the opinion of the BfR that pharmacological reactions to drinks typically containing 50 mg of theanine per 500 milliliters could not be excluded—reactions such as impairment of psychomotor skills and amplification of the sedating effects of alcohol and hypnotics.[36]
In 2006, a study found no consistent, statistically significant treatment-related adverse effects on behavior, morbidity, mortality, body weight, food consumption and efficiency, clinical chemistry, hematology, or urinalysis in rats fed high doses of theanine for 13 weeks.[37] Large studies in humans have not been undertaken; however, several smaller-scale studies (fewer than 100 participants) have shown increased alpha wave generation and lowered anxiety, along with benefits to sleep quality in people with ADHD.[38][39][40]
The combination of theanine and caffeine has been shown to promote faster simple reaction time, faster numeric working memory reaction time and improved sentence verification accuracy.[41][42][43][44]
Theanine has been reported to raise levels of brain serotonin and dopamine, with possible improvement in specific memory and learning tasks.[45]
In brewed teabags
A study of teabags sold in British supermarkets in 2011 found that the teabags containing the most L-theanine per cup (24 mg versus 8 mg per cup) were the lower-quality brands containing black tea, with a supermarket brand of black tea having the highest theanine content. The study demonstrates that brewing time is a major determinant of the amount of l-theanine extracted. Addition of sugar and small quantities of milk make no significant difference, while larger quantities of milk reduced the measured theanine content.[46]
See also
- gamma-Glutamylmethylamide
- Green tea
References
- 1 2 Scheid, L.; Ellinger, S.; Alteheld, B.; Herholz, H.; Ellinger, J.; Henn, T.; Helfrich, H.-P.; Stehle, P. (2012). "Kinetics of L-Theanine Uptake and Metabolism in Healthy Participants Are Comparable after Ingestion of L-Theanine via Capsules and Green Tea". Journal of Nutrition. 142 (12): 2091–2096. doi:10.3945/jn.112.166371. ISSN 0022-3166. PMID 23096008.
- 1 2 3 4 "D-theanine | C7H14N2O3". ChemSpider.com. Retrieved 2015-05-21.
- 1 2 "Theanine". Pubchem Compound. NCBI. Retrieved 21 February 2015.
- ↑ "Components of Gyokuro| IPPODO". Ippodo-tea.co.jp. Retrieved 2015-05-07.
- 1 2 SAKATO Y. J. Agri. Chem. Soc. 1949, 23, 262-7
- ↑ MASON R. Altern. & Complementary Ther. 2001, 7, 91-5
- ↑ "Scientific Opinion on the substantiation of health claims related to L-theanine from Camellia sinensis (L.) Kuntze (Tea) and improvement of cognitive function (ID 1104, 1222, 1600, 1601, 1707, 1935, 2004, 2005), alleviation of psychological stress (ID 1598, 1601), maintenance of normal sleep (ID 1222, 1737, 2004) and reduction of menstrual discomfort (ID 1599) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 | European Food Safety Authority".
- ↑ "EU Register on nutrition and health claims". European Commission. p. 153. Retrieved 2021-08-10.
- ↑ Vuong, Quan V; Bowyer, Michael C; Roach, Paul D (2011). "L-Theanine: Properties, synthesis and isolation from tea". Journal of the Science of Food and Agriculture. 91 (11): 1931–9. doi:10.1002/jsfa.4373. PMID 21735448.
- 1 2 3 Desai, M. J.; Armstrong, D. W. (2004). "Analysis of derivatized and underivatized theanine enantiomers by high-performance liquid chromatography/atmospheric pressure ionization-mass spectrometry". Rapid Communications in Mass Spectrometry. 18 (3): 251–6. Bibcode:2004RCMS...18..251D. doi:10.1002/rcm.1319. PMID 14755608.
- ↑ "How Gyokuro is Processed | IPPODO". Ippodo-tea.co.jp. Archived from the original on 2018-04-25. Retrieved 2015-05-07.
- ↑ Finger, Andreas; Kuhr, Susanne; Engelhardt, Ulrich (1992). "Chromatography of tea constituents". Journal of Chromatography. 624 (1–2): 309–310. doi:10.1016/0021-9673(92)85685-M. PMID 1494009.
- ↑ Casimir, J.; Jadot, J.; Renard, M. (1960). "Séparation et caractérisation de la N-éthyl-γ-glutamine à partir de Xerocomus badius" [Separation and characterization of N-ethyl-γ-glutamine from Xerocomus badius]. Biochimica et Biophysica Acta (in French). 39 (3): 462–8. doi:10.1016/0006-3002(60)90199-2. PMID 13808157.
- ↑ Kurihara, Shigekazu; Shibakusa, Tetsuro; Tanaka, Kenji AK (2013). "Cystine and theanine: Amino acids as oral immunomodulative nutrients". SpringerPlus. 2: 635. doi:10.1186/2193-1801-2-635. PMC 3851524. PMID 24312747.
- ↑ Albrecht, Jan; Sidoryk-Węgrzynowicz, Marta; Zielińska, Magdalena; Aschner, Michael (November 2010). "Roles of glutamine in neurotransmission". Neuron Glia Biology. 6 (4): 263–276. doi:10.1017/S1740925X11000093. ISSN 1741-0533. PMID 22018046.
- 1 2 Yokogoshi, Hidehiko; Kobayashi, Miki; Mochizuki, Mikiko; Terashima, Takehiko (1998). "Effect of theanine, γ-glutamylethylamide, on brain monoamines and striatal dopamine release in conscious rats". Neurochemical Research. 23 (5): 667–73. doi:10.1023/A:1022490806093. PMID 9566605. S2CID 24749717.
- 1 2 3 4 Nathan, Pradeep; Lu, Kristy; Gray, M.; Oliver, C. (2006). "The Neuropharmacology of L-Theanine(N-Ethyl-L-Glutamine)". Journal of Herbal Pharmacotherapy. 6 (2): 21–30. doi:10.1300/J157v06n02_02. PMID 17182482.
- ↑ Kakuda T, Nozawa A, Sugimoto A, Niino H (2002). "Inhibition by theanine of binding of [3H]AMPA, [3H]kainate, and [3H]MDL 105,519 to glutamate receptors". Biosci. Biotechnol. Biochem. 66 (12): 2683–6. doi:10.1271/bbb.66.2683. PMID 12596867. S2CID 26585005.
- 1 2 Kakuda T (2011). "Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction". Pharmacol. Res. 64 (2): 162–8. doi:10.1016/j.phrs.2011.03.010. PMID 21477654.
- 1 2 Kakuda T (2002). "Neuroprotective effects of the green tea components theanine and catechins". Biol. Pharm. Bull. 25 (12): 1513–8. doi:10.1248/bpb.25.1513. PMID 12499631.
- ↑ Sebih, Fatiha; Rousset, Matthieu; Bellahouel, Salima; Rolland, Marc; de Jesus Ferreira, Marie Celeste; Guiramand, Janique; Cohen-Solal, Catherine; Barbanel, Gérard; Cens, Thierry; Abouazza, Mohammed; Tassou, Adrien (2017-08-16). "Characterization of l-Theanine Excitatory Actions on Hippocampal Neurons: Toward the Generation of Novel N-Methyl-d-aspartate Receptor Modulators Based on Its Backbone". ACS Chemical Neuroscience. 8 (8): 1724–1734. doi:10.1021/acschemneuro.7b00036. ISSN 1948-7193. PMID 28511005.
- ↑ Nagasawa K, Aoki H, Yasuda E, Nagai K, Shimohama S, Fujimoto S (2004). "Possible involvement of group I mGluRs in neuroprotective effect of theanine". Biochem. Biophys. Res. Commun. 320 (1): 116–22. doi:10.1016/j.bbrc.2004.05.143. PMID 15207710.
- ↑ Sugiyama T, Sadzuka Y, Tanaka K, Sonobe T (2001). "Inhibition of glutamate transporter by theanine enhances the therapeutic efficacy of doxorubicin". Toxicol. Lett. 121 (2): 89–96. doi:10.1016/s0378-4274(01)00317-4. PMID 11325559.
- ↑ Sugiyama T, Sadzuka Y (2003). "Theanine and glutamate transporter inhibitors enhance the antitumor efficacy of chemotherapeutic agents". Biochim. Biophys. Acta. 1653 (2): 47–59. doi:10.1016/s0304-419x(03)00031-3. PMID 14643924.
- ↑ Narukawa M, Toda Y, Nakagita T, Hayashi Y, Misaka T (2014). "L-Theanine elicits umami taste via the T1R1 + T1R3 umami taste receptor". Amino Acids. 46 (6): 1583–7. doi:10.1007/s00726-014-1713-3. PMID 24633359. S2CID 17380461.
- ↑ Wakabayashi C, Numakawa T, Ninomiya M, Chiba S, Kunugi H (2012). "Behavioral and molecular evidence for psychotropic effects in L-theanine". Psychopharmacology. 219 (4): 1099–109. doi:10.1007/s00213-011-2440-z. PMID 21861094. S2CID 13824013.
- ↑ Yamada T, Terashima T, Wada K, Ueda S, Ito M, Okubo T, Juneja LR, Yokogoshi H (2007). "Theanine, r-glutamylethylamide, increases neurotransmission concentrations and neurotrophin mRNA levels in the brain during lactation". Life Sci. 81 (16): 1247–55. doi:10.1016/j.lfs.2007.08.023. PMID 17904164.
- ↑ Yokogoshi H, Kobayashi M, Mochizuki M, Terashima T (1998). "Effect of theanine, r-glutamylethylamide, on brain monoamines and striatal dopamine release in conscious rats". Neurochem. Res. 23 (5): 667–73. doi:10.1023/A:1022490806093. PMID 9566605. S2CID 24749717.
- ↑ Yokogoshi, Hidehiko; Mochizuki, Mikiko; Saitoh, Kotomi (1998). "Theanine-induced Reduction of Brain Serotonin Concentration in Rats". Bioscience, Biotechnology, and Biochemistry. 62 (4): 816–7. doi:10.1271/bbb.62.816. PMID 9614715.
- ↑ Yokogoshi, Hidehiko; Kato, Yukiko; Sagesaka, Yuko M.; Takihara-Matsuura, Takanobu; Kakuda, Takami; Takeuchi, Naokazu (1995). "Reduction Effect of Theanine on Blood Pressure and Brain 5-Hydroxyindoles in Spontaneously Hypertensive Rats". Bioscience, Biotechnology, and Biochemistry. 59 (4): 615–8. doi:10.1271/bbb.59.615. PMID 7539642.
- ↑ "Theanine Monograph". Natural Standard. Archived from the original on December 24, 2014. Retrieved 30 October 2014.
- ↑ Everett, J.M.; Gunathilake, D.; Dufficy, L.; Roach, P.; Thomas, J.; Upton, D.; Naumovski, N. (2016). "Theanine consumption, stress and anxiety in human clinical trials: A systematic review". Journal of Nutrition & Intermediary Metabolism. 4: 41–42. doi:10.1016/j.jnim.2015.12.308.
- ↑ Williams JL, Everett JM, D'Cunha NM, Sergi D, Georgousopoulou EN, Keegan RJ; et al. (2020). "The Effects of Green Tea Amino Acid L-Theanine Consumption on the Ability to Manage Stress and Anxiety Levels: a Systematic Review". Plant Foods Hum Nutr. 75 (1): 12–23. doi:10.1007/s11130-019-00771-5. PMID 31758301. S2CID 208213702.
The supplementation of L-THE in its pure form at dosages between 200 and 400 mg/day may help reduce stress and anxiety acutely in people undergoing acute stressful situations, but there is insufficient evidence to suggest it assists in the reduction of stress levels in people with chronic conditions. However, the results of this study suggest that L-THE taken during times of heightened acute stress or by individuals with a high propensity for anxiety and stress may exhibit beneficial properties via the increased production of alpha waves and decrease of glutamate in the brain.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ https://www.bfr.bund.de/cm/343/getraenke_mit_isoliertem_l_theanin.pdf
- ↑ Robin B. Kanarek; Harris R. Lieberman (6 October 2011). Diet, Brain, Behavior: Practical Implications. CRC Press. pp. 239–. ISBN 978-1-4398-2156-5.
- ↑ "Getränke mit isoliertem L-Theanin" [Beverages with isolated L-theanine] (PDF) (in German). Bundesinstitut für Risikobewertung. August 2003.
- ↑ Borzelleca, J.F.; Peters, D.; Hall, W. (2006). "A 13-week dietary toxicity and toxicokinetic study with l-theanine in rats". Food and Chemical Toxicology. 44 (7): 1158–66. doi:10.1016/j.fct.2006.03.014. PMID 16759779.
- ↑ Kimura, Kenta; Ozeki, Makoto; Juneja, Lekh Raj; Ohira, Hideki (2007). "L-Theanine reduces psychological and physiological stress responses". Biological Psychology. 74 (1): 39–45. doi:10.1016/j.biopsycho.2006.06.006. PMID 16930802. S2CID 6358088.
- ↑ Lyon, Michael R.; Kapoor, Mahendra P.; Juneja, Lekh R. (2011). "The effects of L-theanine (Suntheanine®) on objective sleep quality in boys with attention deficit hyperactivity disorder (ADHD): a randomized, double-blind, placebo-controlled clinical trial" (PDF). Alternative Medicine Review. 16 (4): 348–54. PMID 22214254. Archived from the original (PDF) on 2017-11-18.
- ↑ Kobayashi, Kanari; Nagato, Yukiko; Aoi, Nobuyuki; Juneja, Lekh Raj; Kim, Mujo; Yamamoto, Takehiko; Sugimoto, Sukeo (1998). "L-テアニンのヒトの脳波に及ぼす影響" [Effects of L-Theanine on the Release of α-Brain Waves in Human Volunteers]. Journal of the Agricultural Chemical Society of Japan (in Japanese). 72 (2): 153–7. doi:10.1271/nogeikagaku1924.72.153.
- ↑ Haskell, Crystal F.; Kennedy, David O.; Milne, Anthea L.; Wesnes, Keith A.; Scholey, Andrew B. (2008). "The effects of l-theanine, caffeine and their combination on cognition and mood". Biological Psychology. 77 (2): 113–22. doi:10.1016/j.biopsycho.2007.09.008. PMID 18006208. S2CID 3772348.
- ↑ Owen, Gail N.; Parnell, Holly; De Bruin, Eveline A.; Rycroft, Jane A. (2008). "The combined effects of L-theanine and caffeine on cognitive performance and mood". Nutritional Neuroscience. 11 (4): 193–8. doi:10.1179/147683008X301513. PMID 18681988. S2CID 46326744.
- ↑ Bryan, Janet (2008). "Psychological effects of dietary components of tea: Caffeine and L-theanine". Nutrition Reviews. 66 (2): 82–90. doi:10.1111/j.1753-4887.2007.00011.x. PMID 18254874.
- ↑ Kelly, Simon P.; Gomez-Ramirez, Manuel; Montesi, Jennifer L.; Foxe, John J. (2008). "L-Theanine and Caffeine in Combination Affect Human Cognition as Evidenced by Oscillatory alpha-Band Activity and Attention Task Performance". The Journal of Nutrition. 138 (8): 1572S–1577S. doi:10.1093/jn/138.8.1572S. PMID 18641209.
- ↑ Nathan, PJ; Lu, K; Gray, M; Oliver, C (2015-04-20). "The neuropharmacology of L-theanine(N-ethyl-L-glutamine): a possible neuroprotective and cognitive enhancing agent". J Herb Pharmacother. 6 (2): 21–30. doi:10.1300/J157v06n02_02. PMID 17182482.
- ↑ Keenan, Emma K.; Finnie, Mike D.A.; Jones, Paul S.; Rogers, Peter J.; Priestley, Caroline M. (2011). "How much theanine in a cup of tea? Effects of tea type and method of preparation". Food Chemistry. 125 (2): 588. doi:10.1016/j.foodchem.2010.08.071.
Further reading
- E.K. Keenan; M.D.A. Finnie; P.S. Jones; P.J. Rogers; C.M. Priestley (2011). "How much theanine in a cup of tea? Effects of tea type and method of preparation". Food Chemistry. 125 (2): 588–594. doi:10.1016/j.foodchem.2010.08.071.
- Y. Orihara; T. Furuya (1990). "Production of theanine and other γ-glutamyl derivatives by Camellia sinensis cultured cells". Plant Cell Reports. 9 (2): 65–68. doi:10.1007/BF00231550. PMID 24226431. S2CID 23515765.